Engineering the flagellar type III secretion system: improving capacity for secretion of recombinant protein
- PMID: 30657054
- PMCID: PMC6337784
- DOI: 10.1186/s12934-019-1058-4
Engineering the flagellar type III secretion system: improving capacity for secretion of recombinant protein
Abstract
Background: Many valuable biopharmaceutical and biotechnological proteins have been produced in Escherichia coli, however these proteins are almost exclusively localised in the cytoplasm or periplasm. This presents challenges for purification, i.e. the removal of contaminating cellular constituents. One solution is secretion directly into the surrounding media, which we achieved via the 'hijack' of the flagellar type III secretion system (FT3SS). Ordinarily flagellar subunits are exported through the centre of the growing flagellum, before assembly at the tip. However, we exploit the fact that in the absence of certain flagellar components (e.g. cap proteins), monomeric flagellar proteins are secreted into the supernatant.
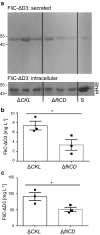
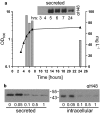
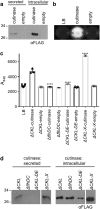
Results: We report the creation and iterative improvement of an E. coli strain, by means of a modified FT3SS and a modular plasmid system, for secretion of exemplar proteins. We show that removal of the flagellin and HAP proteins (FliC and FlgKL) resulted in an optimal prototype. We next developed a high-throughput enzymatic secretion assay based on cutinase. This indicated that removal of the flagellar motor proteins, motAB (to reduce metabolic burden) and protein degradation machinery, clpX (to boost FT3SS levels intracellularly), result in high capacity secretion. We also show that a secretion construct comprising the 5'UTR and first 47 amino acidsof FliC from E. coli (but no 3'UTR) achieved the highest levels of secretion. Upon combination, we show a 24-fold improvement in secretion of a heterologous (cutinase) enzyme over the original strain. This improved strain could export a range of pharmaceutically relevant heterologous proteins [hGH, TrxA, ScFv (CH2)], achieving secreted yields of up to 0.29 mg L-1, in low cell density culture.
Conclusions: We have engineered an E. coli which secretes a range of recombinant proteins, through the FT3SS, to the extracellular media. With further developments, including cell culture process strategies, we envision further improvement to the secreted titre of recombinant protein, with the potential application for protein production for biotechnological purposes.
Keywords: Biotechnology; Flagellar; Recombinant protein secretion; Secretion assay; Strain engineering; Synthetic biology.
Figures





Similar articles
-
Extracellular overexpression of recombinant Thermobifida fusca cutinase by alpha-hemolysin secretion system in E. coli BL21(DE3).Microb Cell Fact. 2012 Jan 12;11:8. doi: 10.1186/1475-2859-11-8. Microb Cell Fact. 2012. PMID: 22239833 Free PMC article.
-
In Vitro Reconstitution of Functional Type III Protein Export and Insights into Flagellar Assembly.mBio. 2018 Jun 26;9(3):e00988-18. doi: 10.1128/mBio.00988-18. mBio. 2018. PMID: 29946050 Free PMC article.
-
Conserved GYXLI Motif of FlhA Is Involved in Dynamic Domain Motions of FlhA Required for Flagellar Protein Export.Microbiol Spectr. 2022 Aug 31;10(4):e0111022. doi: 10.1128/spectrum.01110-22. Epub 2022 Jul 25. Microbiol Spectr. 2022. PMID: 35876582 Free PMC article.
-
Purification of secreted recombinant proteins from Escherichia coli.Bioprocess Technol. 1991;12:163-81. Bioprocess Technol. 1991. PMID: 1367083 Review.
-
Advancements in Escherichia coli secretion systems for enhanced recombinant protein production.World J Microbiol Biotechnol. 2025 Mar 3;41(3):90. doi: 10.1007/s11274-025-04302-0. World J Microbiol Biotechnol. 2025. PMID: 40025370 Review.
Cited by
-
Engineered bacteria as an orally administered anti-viral treatment and immunization system.Gut Microbes. 2025 Dec;17(1):2500056. doi: 10.1080/19490976.2025.2500056. Epub 2025 May 8. Gut Microbes. 2025. PMID: 40340796 Free PMC article.
-
Comparison of Enzyme Secretion and Ferulic Acid Production by Escherichia coli Expressing Different Lactobacillus Feruloyl Esterases.Front Microbiol. 2020 Nov 3;11:568716. doi: 10.3389/fmicb.2020.568716. eCollection 2020. Front Microbiol. 2020. PMID: 33329424 Free PMC article.
-
Functional divergence of flagellar type III secretion system: A case study in a non-flagellated, predatory bacterium.Comput Struct Biotechnol J. 2020 Nov 10;18:3368-3376. doi: 10.1016/j.csbj.2020.10.029. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33294133 Free PMC article.
-
Identification of a new export signal that targets early subunits to the flagellar type III secretion export machinery.mBio. 2024 Mar 13;15(3):e0306723. doi: 10.1128/mbio.03067-23. Epub 2024 Feb 20. mBio. 2024. PMID: 38376149 Free PMC article.
-
Commercialized artemisinin derivatives combined with colistin protect against critical Gram-negative bacterial infection.Commun Biol. 2022 Sep 8;5(1):931. doi: 10.1038/s42003-022-03898-5. Commun Biol. 2022. PMID: 36076060 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- BB/M007197/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F018681/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- CBMNet Business Innovation Voucher/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J016322/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- John Lucas Walker studentship/Department of Pathology, University of Cambridge
LinkOut - more resources
Full Text Sources
Miscellaneous

