The functional role of integrins during intra- and extravasation within the metastatic cascade
- PMID: 30657059
- PMCID: PMC6337777
- DOI: 10.1186/s12943-018-0937-3
The functional role of integrins during intra- and extravasation within the metastatic cascade
Abstract
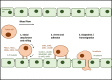
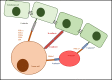
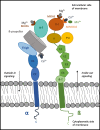
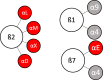
Formation of distant metastases is by far the most common cause of cancer-related deaths. The process of metastasis formation is complex, and within this complex process the formation of migratory cells, the so called epithelial mesenchymal transition (EMT), which enables cancer cells to break loose from the primary tumor mass and to enter the bloodstream, is of particular importance. To break loose from the primary cancer, cancer cells have to down-regulate the cell-to-cell adhesion molecuIes (CAMs) which keep them attached to neighboring cancer cells. In contrast to this downregulation of CAMS in the primary tumor, cancer cells up-regulate other types of CAMs, that enable them to attach to the endothelium in the organ of the future metastasis. During EMT, the expression of cell-to-cell and cell-to-matrix adhesion molecules and their down- and upregulation is therefore critical for metastasis formation. Tumor cells mimic leukocytes to enable transmigration of the endothelial barrier at the metastatic site. The attachment of leukocytes/cancer cells to the endothelium are mediated by several CAMs different from those at the site of the primary tumor. These CAMs and their ligands are organized in a sequential row, the leukocyte adhesion cascade. In this adhesion process, integrins and their ligands are centrally involved in the molecular interactions governing the transmigration. This review discusses the integrin expression patterns found on primary tumor cells and studies whether their expression correlates with tumor progression, metastatic capacity and prognosis. Simultaneously, further possible, but so far unclearly characterized, alternative adhesion molecules and/or ligands, will be considered and emerging therapeutic possibilities reviewed.
Keywords: Cancer; Epithelial mesenchymal transition; Extravasation; Integrin; Integrin inhibitor; Integrin ligands; Leukocyte adhesion cascade; Metastasis; Prognosis; Selectin.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Role of integrins and other cell adhesion molecules in tumor progression and metastasis.Lab Invest. 1993 Jan;68(1):4-17. Lab Invest. 1993. PMID: 8423675 Review.
-
Interplay of integrins and selectins in metastasis.Mol Oncol. 2025 Jun;19(6):1582-1611. doi: 10.1002/1878-0261.70026. Epub 2025 May 6. Mol Oncol. 2025. PMID: 40327521 Free PMC article. Review.
-
alpha 4 integrins and tumor metastasis.Curr Top Microbiol Immunol. 1998;231:125-41. doi: 10.1007/978-3-642-71987-5_8. Curr Top Microbiol Immunol. 1998. PMID: 9479864 Review.
-
Endothelial cell adhesion molecules and cancer progression.Curr Med Chem. 2007;14(4):377-86. doi: 10.2174/092986707779941032. Curr Med Chem. 2007. PMID: 17305540 Review.
-
S-Nitrosocaptopril prevents cancer metastasis in vivo by creating the hostile bloodstream microenvironment against circulating tumor cells.Pharmacol Res. 2019 Jan;139:535-549. doi: 10.1016/j.phrs.2018.10.020. Epub 2018 Oct 23. Pharmacol Res. 2019. PMID: 30366102
Cited by
-
Identification of lymphocyte cell-specific protein-tyrosine kinase (LCK) as a driver for invasion and migration of oral cancer by tumor heterogeneity exploitation.Mol Cancer. 2021 Jun 11;20(1):88. doi: 10.1186/s12943-021-01384-w. Mol Cancer. 2021. PMID: 34116687 Free PMC article.
-
Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities.Cell Commun Signal. 2023 Sep 28;21(1):266. doi: 10.1186/s12964-023-01264-4. Cell Commun Signal. 2023. PMID: 37770930 Free PMC article. Review.
-
Role of NO and S-nitrosylation in the Expression of Endothelial Adhesion Proteins That Regulate Leukocyte and Tumor Cell Adhesion.Front Physiol. 2020 Nov 13;11:595526. doi: 10.3389/fphys.2020.595526. eCollection 2020. Front Physiol. 2020. PMID: 33281627 Free PMC article. Review.
-
IBSP, a potential recurrence biomarker, promotes the progression of colorectal cancer via Fyn/β-catenin signaling pathway.Cancer Med. 2021 Jun;10(12):4030-4045. doi: 10.1002/cam4.3959. Epub 2021 May 13. Cancer Med. 2021. PMID: 33987980 Free PMC article.
-
Role of c-Met/β1 integrin complex in the metastatic cascade in breast cancer.JCI Insight. 2021 Jun 22;6(12):e138928. doi: 10.1172/jci.insight.138928. JCI Insight. 2021. PMID: 34003803 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

