Preventive action of benztropine on platinum-induced peripheral neuropathies and tumor growth
- PMID: 30657060
- PMCID: PMC6337872
- DOI: 10.1186/s40478-019-0657-y
Preventive action of benztropine on platinum-induced peripheral neuropathies and tumor growth
Abstract
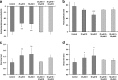
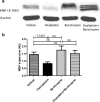
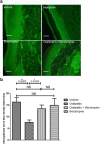
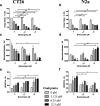
The endogenous cholinergic system plays a key role in neuronal cells, by suppressing neurite outgrowth and myelination and, in some cancer cells, favoring tumor growth. Platinum compounds are widely used as part of first line conventional cancer chemotherapy; their efficacy is however limited by peripheral neuropathy as a major side-effect. In a multiple sclerosis mouse model, benztropine, that also acts as an anti-histamine and a dopamine re-uptake inhibitor, induced the differentiation of oligodendrocytes through M1 and M3 muscarinic receptors and enhanced re-myelination. We have evaluated whether benztropine can increase anti-tumoral efficacy of oxaliplatin, while preventing its neurotoxicity.We showed that benztropine improves acute and chronic clinical symptoms of oxaliplatin-induced peripheral neuropathies in mice. Sensory alterations detected by electrophysiology in oxaliplatin-treated mice were consistent with a decreased nerve conduction velocity and membrane hyperexcitability due to alterations in the density and/or functioning of both sodium and potassium channels, confirmed by action potential analysis from ex-vivo cultures of mouse dorsal root ganglion sensory neurons using whole-cell patch-clamp. These alterations were all prevented by benztropine. In oxaliplatin-treated mice, MBP expression, confocal and electronic microscopy of the sciatic nerves revealed a demyelination and confirmed the alteration of the myelinated axons morphology when compared to animals injected with oxaliplatin plus benztropine. Benztropine also prevented the decrease in neuronal density in the paws of mice injected with oxaliplatin. The neuroprotection conferred by benztropine against chemotherapeutic drugs was associated with a lower expression of inflammatory cytokines and extended to diabetic-induced peripheral neuropathy in mice.Mice receiving benztropine alone presented a lower tumor growth when compared to untreated animals and synergized the anti-tumoral effect of oxaliplatin, a phenomenon explained at least in part by benztropine-induced ROS imbalance in tumor cells.This report shows that blocking muscarinic receptors with benztropine prevents peripheral neuropathies and increases the therapeutic index of oxaliplatin. These results can be rapidly transposable to patients as benztropine is currently indicated in Parkinson's disease in the United States.
Keywords: Benztropine; Muscarinic receptors; Myelin; Oxaliplatin; Peripheral neuropathies.
Conflict of interest statement
Competing interests
Tânia Cristina Gonçalves was an employee of Sanofi (and a Sanofi hareholder via participation/profit-sharing) when these studies were conducted. All other co-authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures








Similar articles
-
Neuroprotective effect of alogliptin on oxaliplatin-induced peripheral neuropathy in vivo and in vitro.Sci Rep. 2020 Apr 21;10(1):6734. doi: 10.1038/s41598-020-62738-w. Sci Rep. 2020. PMID: 32317735 Free PMC article.
-
Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice.Mol Pain. 2011 Apr 26;7:29. doi: 10.1186/1744-8069-7-29. Mol Pain. 2011. PMID: 21521528 Free PMC article.
-
Carvedilol prevents functional deficits in peripheral nerve mitochondria of rats with oxaliplatin-evoked painful peripheral neuropathy.Toxicol Appl Pharmacol. 2017 May 1;322:97-103. doi: 10.1016/j.taap.2017.03.009. Epub 2017 Mar 9. Toxicol Appl Pharmacol. 2017. PMID: 28286117
-
[Role of Transient Receptor Potential Channels in Paclitaxel- and Oxaliplatin-induced Peripheral Neuropathy].Yakugaku Zasshi. 2016;136(2):287-96. doi: 10.1248/yakushi.15-00214. Yakugaku Zasshi. 2016. PMID: 26831807 Review. Japanese.
-
Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms?Neurosci Lett. 2015 Jun 2;596:90-107. doi: 10.1016/j.neulet.2014.10.014. Epub 2014 Oct 22. Neurosci Lett. 2015. PMID: 25459280 Review.
Cited by
-
The past, present and future of anticholinergic drugs.Ther Adv Psychopharmacol. 2023 Sep 8;13:20451253231176375. doi: 10.1177/20451253231176375. eCollection 2023. Ther Adv Psychopharmacol. 2023. PMID: 37701889 Free PMC article. Review.
-
Management of Oxaliplatin-Induced Peripheral Sensory Neuropathy.Cancers (Basel). 2020 May 27;12(6):1370. doi: 10.3390/cancers12061370. Cancers (Basel). 2020. PMID: 32471028 Free PMC article. Review.
-
A muscarinic receptor antagonist reverses multiple indices of diabetic peripheral neuropathy: preclinical and clinical studies using oxybutynin.Acta Neuropathol. 2024 Mar 25;147(1):60. doi: 10.1007/s00401-024-02710-4. Acta Neuropathol. 2024. PMID: 38526612 Clinical Trial.
-
Oxaliplatin Depolarizes the IB4- Dorsal Root Ganglion Neurons to Drive the Development of Neuropathic Pain Through TRPM8 in Mice.Front Mol Neurosci. 2021 Jun 4;14:690858. doi: 10.3389/fnmol.2021.690858. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34149356 Free PMC article.
-
Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy.Front Immunol. 2021 Feb 4;11:626687. doi: 10.3389/fimmu.2020.626687. eCollection 2020. Front Immunol. 2021. PMID: 33613570 Free PMC article. Review.
References
-
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A, Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

