The Biology of Streptococcus mutans
- PMID: 30657107
- PMCID: PMC6615571
- DOI: 10.1128/microbiolspec.GPP3-0051-2018
The Biology of Streptococcus mutans
Abstract
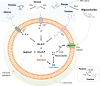
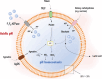
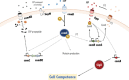
As a major etiological agent of human dental caries, Streptococcus mutans resides primarily in biofilms that form on the tooth surfaces, also known as dental plaque. In addition to caries, S. mutans is responsible for cases of infective endocarditis with a subset of strains being indirectly implicated with the onset of additional extraoral pathologies. During the past 4 decades, functional studies of S. mutans have focused on understanding the molecular mechanisms the organism employs to form robust biofilms on tooth surfaces, to rapidly metabolize a wide variety of carbohydrates obtained from the host diet, and to survive numerous (and frequent) environmental challenges encountered in oral biofilms. In these areas of research, S. mutans has served as a model organism for ground-breaking new discoveries that have, at times, challenged long-standing dogmas based on bacterial paradigms such as Escherichia coli and Bacillus subtilis. In addition to sections dedicated to carbohydrate metabolism, biofilm formation, and stress responses, this article discusses newer developments in S. mutans biology research, namely, how S. mutans interspecies and cross-kingdom interactions dictate the development and pathogenic potential of oral biofilms and how next-generation sequencing technologies have led to a much better understanding of the physiology and diversity of S. mutans as a species.
Figures

References
-
- Clarke JK. 1924. On the bacterial factor in the etiology of dental caries. Br J Exp Pathol 5:141–147. [PubMed]
-
- Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99:14434–14439 10.1073/pnas.172501299. [PubMed] - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

