Invasive Nontyphoidal Salmonella Disease in Africa
- PMID: 30657108
- PMCID: PMC11573285
- DOI: 10.1128/ecosalplus.ESP-0007-2018
Invasive Nontyphoidal Salmonella Disease in Africa
Abstract
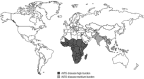
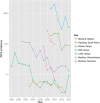
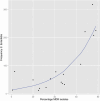
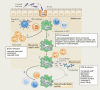
Nontyphoidal salmonellae (NTS) are a major cause of invasive (iNTS) disease in sub-Saharan Africa, manifesting as bacteremia and meningitis. Available epidemiological data indicate that iNTS disease is endemic in much of the region. Antimicrobial resistance is common and case fatality rates are high. There are well-characterized clinical associations with iNTS disease, including young age, HIV infection, malaria, malnutrition, anemia, and sickle cell disease. However, the clinical presentation of iNTS disease is often with fever alone, so clinical diagnosis is impossible without blood culture confirmation. No vaccine is currently available, making this a priority area for global health research. Over the past ten years, it has emerged that iNTS disease in Africa is caused by distinct pathovars of Salmonella Typhimurium, belonging to sequence type ST313, and Salmonella Enteritidis. These are characterized by genome degradation and appear to be adapting to an invasive lifestyle. Investigation of rare patients with primary immunodeficiencies has suggested a key role for interferon gamma-mediated immunity in host defense against NTS. This concept has been supported by recent population-based host genetic studies in African children. In contrast, immunoepidemiological studies from Africa indicate an important role for antibody for protective immunity, supporting the development of antibody-inducing vaccines against iNTS disease. With candidate O-antigen-based vaccines due to enter clinical trials in the near future, research efforts should focus on understanding the relative contributions of antibody and cell-mediated immunity to protection against iNTS disease in humans.
Figures
References
-
- Doolittle RF, Feng DF, Tsang S, Cho G, Little E. 1996. Determining divergence times of the major kingdoms of living organisms with a protein clock. Science 271:470–477. - PubMed
-
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N Engl J Med 347:1770–1782. - PubMed
-
- Hohmann EL. 2001. Nontyphoidal salmonellosis. Clin Infect Dis 32:263–269. - PubMed
-
- Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, Cieslak PR, Deneen VC, Tauxe RV, Emerging Infections Program FoodNet Working Group. 2004. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis 38(Suppl 3):S127–S134. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

