How to Conduct Clinical Trials in Children: A Tutorial
- PMID: 30657253
- PMCID: PMC6510379
- DOI: 10.1111/cts.12615
How to Conduct Clinical Trials in Children: A Tutorial
Abstract
Despite a growing interest in, and commitment to, implementing pediatric clinical trials, approximately one in every five trials in children fails because of inappropriate study design, suboptimal experiment planning, or inadequate participant enrollment. This tutorial, presented from the perspectives of seasoned pediatric investigators, an experienced research coordinator, and an established pediatric clinical trials network, is designed to provide practical guidance for successfully implementing pediatric clinical trials at an academic center or another comparable institution.
© 2019 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors, currently or in the past, have received funding support from the Pediatric Trials Network (PTN;
Figures

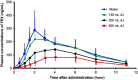


Comment in
-
Involve Children and Parents in Clinical Studies.Clin Transl Sci. 2020 Jan;13(1):11-13. doi: 10.1111/cts.12696. Epub 2019 Oct 24. Clin Transl Sci. 2020. PMID: 31647179 Free PMC article. No abstract available.
References
-
- Tolbert, J. , Golman, J. , Kauffmann, R. & Abdel‐Rahman, S.M. The creating hope act: what is old is new again. Pediatr. Health Med. Ther. 5, 49–57 (2014).
-
- Abdel‐Rahman, S.M. , Reed, M.D. , Wells, T.G. & Kearns, G.L. Considerations in the rational design and conduct of phase I/II pediatric clinical trials: avoiding the problems and pitfalls. Clin. Pharm. Ther. 81, 483–494 (2007). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

