Tripping the light fantastic in membrane redox biology: linking dynamic structures to function in ER electron transfer chains
- PMID: 30657259
- PMCID: PMC6563164
- DOI: 10.1111/febs.14757
Tripping the light fantastic in membrane redox biology: linking dynamic structures to function in ER electron transfer chains
Abstract
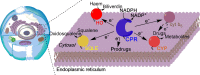
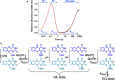
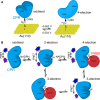
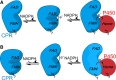
How the dynamics of proteins assist catalysis is a contemporary issue in enzymology. In particular, this holds true for membrane-bound enzymes, where multiple structural, spectroscopic and biochemical approaches are needed to build up a comprehensive picture of how dynamics influence enzyme reaction cycles. Of note are the recent studies of cytochrome P450 reductases (CPR)-P450 (CYP) endoplasmic reticulum redox chains, showing the relationship between dynamics and electron flow through flavin and haem redox centres and the impact this has on monooxygenation chemistry. These studies have led to deeper understanding of mechanisms of electron flow, including the timing and control of electron delivery to protein-bound cofactors needed to facilitate CYP-catalysed reactions. Individual and multiple component systems have been used to capture biochemical behaviour and these have led to the emergence of more integrated models of catalysis. Crucially, the effects of membrane environment and composition on reaction cycle chemistry have also been probed, including effects on coenzyme binding/release, thermodynamic control of electron transfer, conformational coupling between partner proteins and vectorial versus 'off pathway' electron flow. Here, we review these studies and discuss evidence for the emergence of dynamic structural models of electron flow along human microsomal CPR-P450 redox chains.
Keywords: cytochrome P450; cytochrome P450 reductase; electron transfer chemistry; membrane protein; protein domain dynamics.
© 2019 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Henzler‐Wildman KA, Lei M, Thai V, Kerns SJ, Karplus M & Kern D (2007) A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature 450, 913–916. - PubMed
-
- Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto‐Kihara A, Ueda I, Yanagida T, Wada Y & Futai M (1999) Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 286, 1722–1724. - PubMed
-
- Henzler‐Wildman KA, Thai V, Lei M, Ott M, Wolf‐Watz M, Fenn T, Pozharski E, Wilson MA, Petsko GA, Karplus M et al (2007) Intrinsic motions along an enzymatic reaction trajectory. Nature 450, 838–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

