Analysis of Adjuvant Endocrine Therapy in Practice From Electronic Health Record Data of Patients With Breast Cancer
- PMID: 30657375
- PMCID: PMC7010428
- DOI: 10.1200/CCI.16.00044
Analysis of Adjuvant Endocrine Therapy in Practice From Electronic Health Record Data of Patients With Breast Cancer
Abstract
Purpose: Adjuvant endocrine therapy is a long-term drug therapy prescribed to prevent recurrence of hormone receptor-positive breast cancer. Data on adjuvant endocrine therapy are reported though clinical trials, which may differ from treatment practice and outcomes in the general population of patients with breast cancer. With secondary use of electronic health record (EHR) data, we summarize adjuvant endocrine treatment practice and outcomes in real-world settings.
Methods: We analyzed treatment data derived from EHR data on 1,587 patients with stage I to III breast cancer at a National Cancer Institute-designated comprehensive cancer center to learn the frequencies of real-world adjuvant endocrine drug switches and discontinuation and to explore the potential cause for drug switches and discontinuation from medical records. We measured rates of drug use, drug switches, early drug discontinuation, adverse events, recurrence, and death. We also measured adverse events and change in menopause status as potential causes for drug switch and discontinuation.
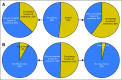
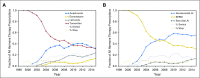
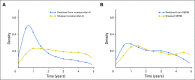
Results: Within the study population, approximately 49% of patients were lost to follow-up or did not complete adjuvant treatment through 5 years. Fifty-two percent of patients switched to a different endocrine therapy drug during their treatment. We found that age is correlated with drug switches and that adverse events are correlated with drug switches and discontinuation. We also found that patients who switched to an alternative endocrine therapy during treatment were more likely to complete 5 years of treatment.
Conclusion: This study describes long-term adjuvant endocrine treatment in real-world settings and demonstrates the ability to leverage longitudinal EHR data to characterize oral medication treatment patterns in patients with cancer.
Conflict of interest statement
Morgan Harrell
No relationship to disclose
Daniel Fabbri
Mia Levy
Figures
References
-
- Rugo HS, Rumble RB, Macrae E, et al. : Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 34:3069-3103, 2016 - PubMed
-
- Hershman DL: Perfecting breast-cancer treatment: Incremental gains and musculoskeletal pains. N Engl J Med 372:477-478, 2015 - PubMed
-
- Howell A, Cuzick J, Baum M, et al. : Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60-62, 2005 - PubMed
-
- Fisher B, Costantino J, Redmond C, et al. : A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 320:479-484, 1989 - PubMed
-
- Mouridsen H, Chaudri-Ross HA: Efficacy of first-line letrozole versus tamoxifen as a function of age in postmenopausal women with advanced breast cancer. Oncologist 9:497-506, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

