Synthesis and Characterization of Site-Specific O6 -Alkylguanine DNA-Alkyl Transferase-Oligonucleotide Crosslinks
- PMID: 30657645
- PMCID: PMC6504252
- DOI: 10.1002/cpnc.74
Synthesis and Characterization of Site-Specific O6 -Alkylguanine DNA-Alkyl Transferase-Oligonucleotide Crosslinks
Abstract
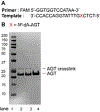
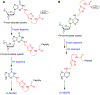
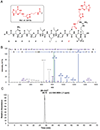
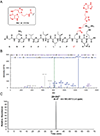
O6 -Alkylguanine DNA-alkyltransferase (AGT), a DNA repair protein, can form crosslinks with DNA. The AGT-DNA crosslinks are known to be mutagenic when AGT is heterologously expressed in Escherichia coli, as well as in mammalian cells. To understand the biological consequences, reliable access to AGT-oligonucleotide crosslinks is needed. This article describes the synthesis and characterization of site-specific AGT-oligonucleotide crosslinks at the N2-position of deoxyguanosine and N6-position of deoxyadenosine. We developed a post-oligomerization strategy for the synthesis of propargyl-modified oligonucleotides. Copper-catalyzed azide-alkyne cycloaddition was used as a key step to obtain the iodoacetamide-linked oligonucleotides, which serve as good electrophiles for the crosslinking reaction with cysteine-145 of the active site of AGT. Trypsinization of AGT and hydrolysis of oligonucleotides, combined with analysis by liquid chromatography-tandem mass spectrometry, was utilized to confirm the nucleobase-adducted peptides. This method provides a useful strategy for the synthesis and characterization of site-specific DNA-protein crosslinks, which can be further used to understand proteolytic degradation-coupled DNA repair mechanisms. © 2019 by John Wiley & Sons, Inc.
Keywords: DNA-protein crosslinks; O6-alkylguanine DNA-alkyltransferase; copper-catalyzed azide-alkyne cycloaddition; oligonucleotides; post-oligomerization; proteomics.
© 2019 John Wiley & Sons, Inc.
Figures
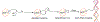



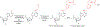


Similar articles
-
Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane.Chem Res Toxicol. 2006 May;19(5):645-54. doi: 10.1021/tx0600088. Chem Res Toxicol. 2006. PMID: 16696566 Free PMC article.
-
Influence of the Expression Level of O6-Alkylguanine-DNA Alkyltransferase on the Formation of DNA Interstrand Crosslinks Induced by Chloroethylnitrosoureas in Cells: A Quantitation Using High-Performance Liquid Chromatography-Mass Spectrometry.PLoS One. 2015 Mar 23;10(3):e0121225. doi: 10.1371/journal.pone.0121225. eCollection 2015. PLoS One. 2015. PMID: 25799182 Free PMC article.
-
Function of domains of human O6-alkylguanine-DNA alkyltransferase.Biochemistry. 2005 Nov 22;44(46):15396-405. doi: 10.1021/bi051460d. Biochemistry. 2005. PMID: 16285744
-
Conserved structural motifs governing the stoichiometric repair of alkylated DNA by O(6)-alkylguanine-DNA alkyltransferase.Mutat Res. 2000 Aug 30;460(3-4):151-63. doi: 10.1016/s0921-8777(00)00024-0. Mutat Res. 2000. PMID: 10946226 Review.
-
Principles of covalent binding of reactive metabolites and examples of activation of bis-electrophiles by conjugation.Arch Biochem Biophys. 2005 Jan 15;433(2):369-78. doi: 10.1016/j.abb.2004.07.035. Arch Biochem Biophys. 2005. PMID: 15581593 Review.
Cited by
-
DNA polymerases η and κ bypass N2-guanine-O6-alkylguanine DNA alkyltransferase cross-linked DNA-peptides.J Biol Chem. 2021 Oct;297(4):101124. doi: 10.1016/j.jbc.2021.101124. Epub 2021 Aug 28. J Biol Chem. 2021. PMID: 34461101 Free PMC article.
-
Enzymatic bypass of an N6-deoxyadenosine DNA-ethylene dibromide-peptide cross-link by translesion DNA polymerases.J Biol Chem. 2021 Jan-Jun;296:100444. doi: 10.1016/j.jbc.2021.100444. Epub 2021 Feb 20. J Biol Chem. 2021. PMID: 33617883 Free PMC article.
References
-
- Barker S, Weinfeld M, & Murray D (2005). DNA–protein crosslinks: their induction, repair, and biological consequences. Mutat. Res. Rev. Mutat. Res, 589, 111–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

