Effective Fungal Spore Inactivation with an Environmentally Friendly Approach Based on Atmospheric Pressure Air Plasma
- PMID: 30657659
- PMCID: PMC6727216
- DOI: 10.1021/acs.est.8b05386
Effective Fungal Spore Inactivation with an Environmentally Friendly Approach Based on Atmospheric Pressure Air Plasma
Abstract
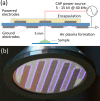
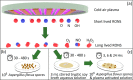
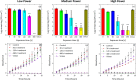
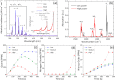
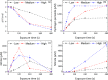
Fungal contamination of surfaces is a global burden, posing a major environmental and public health challenge. A wide variety of antifungal chemical agents are available; however, the side effects of the use of these disinfectants often result in the generation of toxic residues raising major environmental concerns. Herein, atmospheric pressure air plasma generated by a surface barrier discharge (SBD) is presented as an innovative green chemical method for fungal inactivation, with the potential to become an effective replacement for conventional chemical disinfection agents, such as Virkon. Using Aspergillus flavus spores as a target organism, a comparison of plasma based decontamination techniques is reported, highlighting their respective efficiencies and uncovering their underpining inactivation pathways. Tests were performed using both direct gaseous plasma treatment and an indirect treatment using a plasma activated aqueous broth solution (PAB). Concentrations of gaseous ozone and nitrogen oxides were determined with Fourier-transform infrared spectroscopy (FTIR) and Optical emission spectroscopy (OES), whereas hydrogen peroxides, nitrites, nitrates, and pH were measured in PAB. It is demonstrated that direct exposure to the gaseous plasma effluent exhibited superior decontamination efficiency and eliminated spores more effectively than Virkon, a finding attributed to the production of a wide variety of reactive oxygen and nitrogen species within the plasma.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Nelson J. H. Production of blue cheese flavor via submerged fermentation by Penicillium roqueforti. J. Agric. Food Chem. 1970, 18 (4), 567–569. 10.1021/jf60170a024. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

