Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon
- PMID: 30657788
- PMCID: PMC6355048
- DOI: 10.1371/journal.ppat.1007507
Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon
Abstract
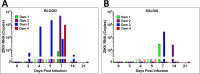
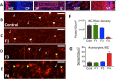
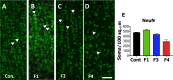
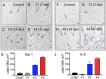
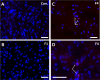
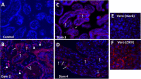
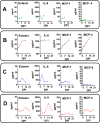
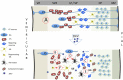
Zika virus (ZIKV) infection during pregnancy in humans is associated with an increased incidence of congenital anomalies including microcephaly as well as fetal death and miscarriage and collectively has been referred to as Congenital Zika Syndrome (CZS). Animal models for ZIKV infection in pregnancy have been developed including mice and non-human primates (NHPs). In macaques, fetal CZS outcomes from maternal ZIKV infection range from none to significant. In the present study we develop the olive baboon (Papio anubis), as a model for vertical transfer of ZIKV during pregnancy. Four mid-gestation, timed-pregnant baboons were inoculated with the French Polynesian ZIKV isolate (104 ffu). This study specifically focused on the acute phase of vertical transfer. Dams were terminated at 7 days post infection (dpi; n = 1), 14 dpi (n = 2) and 21 dpi (n = 1). All dams exhibited mild to moderate rash and conjunctivitis. Viremia peaked at 5-7 dpi with only one of three dams remaining mildly viremic at 14 dpi. An anti-ZIKV IgM response was observed by 14 dpi in all three dams studied to this stage, and two dams developed a neutralizing IgG response by either 14 dpi or 21 dpi, the latter included transfer of the IgG to the fetus (cord blood). A systemic inflammatory response (increased IL2, IL6, IL7, IL15, IL16) was observed in three of four dams. Vertical transfer of ZIKV to the placenta was observed in three pregnancies (n = 2 at 14 dpi and n = 1 at 21 dpi) and ZIKV was detected in fetal tissues in two pregnancies: one associated with fetal death at ~14 dpi, and the other in a viable fetus at 21 dpi. ZIKV RNA was detected in the fetal cerebral cortex and other tissues of both of these fetuses. In the fetus studied at 21 dpi with vertical transfer of virus to the CNS, the frontal cerebral cortex exhibited notable defects in radial glia, radial glial fibers, disorganized migration of immature neurons to the cortical layers, and signs of pathology in immature oligodendrocytes. In addition, indices of pronounced neuroinflammation were observed including astrogliosis, increased microglia and IL6 expression. Of interest, in one fetus examined at 14 dpi without detection of ZIKV RNA in brain and other fetal tissues, increased neuroinflammation (IL6 and microglia) was observed in the cortex. Although the placenta of the 14 dpi dam with fetal death showed considerable pathology, only minor pathology was noted in the other three placentas. ZIKV was detected immunohistochemically in two placentas (14 dpi) and one placenta at 21 dpi but not at 7 dpi. This is the first study to examine the early events of vertical transfer of ZIKV in a NHP infected at mid-gestation. The baboon thus represents an additional NHP as a model for ZIKV induced brain pathologies to contrast and compare to humans as well as other NHPs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46(5):521–34. Epub 1952/09/01. . - PubMed
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46(5):509–20. Epub 1952/09/01. . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

