Outcomes of Hypothermia in Addition to Decompressive Hemicraniectomy in Treatment of Malignant Middle Cerebral Artery Stroke: A Randomized Clinical Trial
- PMID: 30657812
- PMCID: PMC6515837
- DOI: 10.1001/jamaneurol.2018.4822
Outcomes of Hypothermia in Addition to Decompressive Hemicraniectomy in Treatment of Malignant Middle Cerebral Artery Stroke: A Randomized Clinical Trial
Erratum in
-
Errors in Multiple Sections.JAMA Neurol. 2019 May 1;76(5):626. doi: 10.1001/jamaneurol.2019.0131. JAMA Neurol. 2019. PMID: 30801622 Free PMC article. No abstract available.
Abstract
Importance: Moderate hypothermia in addition to early decompressive hemicraniectomy has been suggested to further reduce mortality and improve functional outcome in patients with malignant middle cerebral artery (MCA) stroke.
Objective: To investigate whether moderate hypothermia vs standard treatment after early hemicraniectomy reduces mortality at day 14 in patients with malignant MCA stroke.
Design, setting, and participants: This randomized clinical trial recruited patients from August 2011 through September 2015 at 6 German university hospitals with dedicated neurointensive care units. Of the patients treated with hemicraniectomy and assessed for eligibility, patients were randomly assigned to either standard care or moderate hypothermia. Data analysis was completed from December 2016 to June 2018.
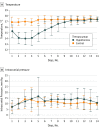
Interventions: Moderate hypothermia (temperature, 33.0 ± 1.0°C) was maintained for at least 72 hours immediately after hemicraniectomy.
Main outcomes and measures: The primary outcome was mortality rate at day 14 compared with the Fisher exact test and expressed as odds ratio (ORs) with 95% CIs. Rates of patients with serious adverse events were estimated for the period of the first 14 days after hemicraniectomy and 12 months of follow-up. Secondary outcome measures included functional outcome at 12 months.
Results: Of the 50 study participants, 24 were assigned to standard care and 26 to moderate hypothermia. Twenty-eight were male (56%); the mean (SD) patient age was 51.3 (6.6) years. Recruitment was suspended for safety concerns: 12 of 26 patients (46%) in the hypothermia group and 7 of 24 patients (29%) receiving standard care had at least 1 serious adverse event within 14 days (OR, 2.05 [95% CI, 0.56-8.00]; P = .26); after 12 months, rates of serious adverse events were 80% (n = 20 of 25) in the hypothermia group and 43% (n = 10 of 23) in the standard care group (hazard ratio, 2.54 [95% CI, 1.29-5.00]; P = .005). The mortality rate at day 14 was 19% (5 of 26 patients) in the hypothermia group and 13% (3 of 24 patients) in the group receiving standard care (OR, 1.65 [95% CI, 0.28-12.01]; P = .70). There was no significant difference regarding functional outcome after 12 months of follow-up.
Interpretation: In patients with malignant MCA stroke, moderate hypothermia early after hemicraniectomy did not improve mortality and functional outcome compared with standard care, but may cause serious harm in this specific setting.
Trial registration: http://www.drks.de, identifier DRKS00000623.
Conflict of interest statement
Figures

Comment in
-
Hypothermia after decompressive hemicraniectomy in treatment of malignant middle cerebral artery stroke: comment on the randomized clinical trial.Crit Care. 2019 May 9;23(1):164. doi: 10.1186/s13054-019-2452-3. Crit Care. 2019. PMID: 31072354 Free PMC article. No abstract available.
Similar articles
-
DEcompressive surgery Plus hypoTHermia for Space-Occupying Stroke (DEPTH-SOS): a protocol of a multicenter randomized controlled clinical trial and a literature review.Int J Stroke. 2013 Jul;8(5):383-7. doi: 10.1111/ijs.12086. Int J Stroke. 2013. PMID: 23782729 Review.
-
Decompressive craniectomy combined with mild hypothermia in patients with large hemispheric infarction: a randomized controlled trial.BMC Neurol. 2021 Mar 12;21(1):114. doi: 10.1186/s12883-021-02142-7. BMC Neurol. 2021. PMID: 33711963 Free PMC article. Clinical Trial.
-
Large diameter hemicraniectomy does not improve long-term outcome in malignant infarction.J Neurol. 2023 Aug;270(8):4080-4089. doi: 10.1007/s00415-023-11766-3. Epub 2023 May 10. J Neurol. 2023. PMID: 37162579 Free PMC article. Clinical Trial.
-
Nationwide survey of decompressive hemicraniectomy for malignant middle cerebral artery infarction in Japan.World Neurosurg. 2014 Dec;82(6):1158-63. doi: 10.1016/j.wneu.2014.07.015. Epub 2014 Jul 18. World Neurosurg. 2014. PMID: 25045787
-
Decompressive Hemicraniectomy in the Treatment of Malignant Middle Cerebral Artery Infarction: A Meta-Analysis.World Neurosurg. 2019 Mar;123:8-16. doi: 10.1016/j.wneu.2018.11.176. Epub 2018 Nov 27. World Neurosurg. 2019. PMID: 30500591
Cited by
-
Hypothermia after decompressive hemicraniectomy in treatment of malignant middle cerebral artery stroke: comment on the randomized clinical trial.Crit Care. 2019 May 9;23(1):164. doi: 10.1186/s13054-019-2452-3. Crit Care. 2019. PMID: 31072354 Free PMC article. No abstract available.
-
Guideline-recommended basic parameter adherence in neurocritical care stroke patients: Observational multicenter individual participant data analysis.Eur Stroke J. 2025 Jun;10(2):504-512. doi: 10.1177/23969873241289360. Epub 2024 Oct 13. Eur Stroke J. 2025. PMID: 39397354 Free PMC article.
-
Fluid Balance Variations During the Early Phase of Large Hemispheric Stroke Are Associated With Patients' Functional Outcome.Front Neurol. 2019 Jul 3;10:720. doi: 10.3389/fneur.2019.00720. eCollection 2019. Front Neurol. 2019. PMID: 31333571 Free PMC article.
-
Neuroimmune mechanisms and therapies mediating post-ischaemic brain injury and repair.Nat Rev Neurosci. 2023 May;24(5):299-312. doi: 10.1038/s41583-023-00690-0. Epub 2023 Mar 27. Nat Rev Neurosci. 2023. PMID: 36973481 Review.
-
Therapeutic hypothermia for acute ischemic stroke: from preclinical studies to clinical trials.Sci China Life Sci. 2025 May 30. doi: 10.1007/s11427-024-2738-2. Online ahead of print. Sci China Life Sci. 2025. PMID: 40459691 Review.

