Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial
- PMID: 30657853
- PMCID: PMC6931268
- DOI: 10.1093/annonc/mdy545
Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial
Abstract
Background: In KEYNOTE-010, pembrolizumab versus docetaxel improved overall survival (OS) in patients with programmed death-1 protein (PD)-L1-positive advanced non-small-cell lung cancer (NSCLC). A prespecified exploratory analysis compared outcomes in patients based on PD-L1 expression in archival versus newly collected tumor samples using recently updated survival data.
Patients and methods: PD-L1 was assessed centrally by immunohistochemistry (22C3 antibody) in archival or newly collected tumor samples. Patients received pembrolizumab 2 or 10 mg/kg Q3W or docetaxel 75 mg/m2 Q3W for 24 months or until progression/intolerable toxicity/other reason. Response was assessed by RECIST v1.1 every 9 weeks, survival every 2 months. Primary end points were OS and progression-free survival (PFS) in tumor proportion score (TPS) ≥50% and ≥1%; pembrolizumab doses were pooled in this analysis.
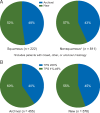
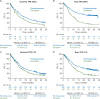
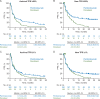
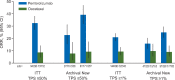
Results: At date cut-off of 24 March 2017, median follow-up was 31 months (range 23-41) representing 18 additional months of follow-up from the primary analysis. Pembrolizumab versus docetaxel continued to improve OS in patients with previously treated, PD-L1-expressing advanced NSCLC; hazard ratio (HR) was 0.66 [95% confidence interval (CI): 0.57, 0.77]. Of 1033 patients analyzed, 455(44%) were enrolled based on archival samples and 578 (56%) on newly collected tumor samples. Approximately 40% of archival samples and 45% of newly collected tumor samples were PD-L1 TPS ≥50%. For TPS ≥50%, the OS HRs were 0.64 (95% CI: 0.45, 0.91) and 0.40 (95% CI: 0.28, 0.56) for archival and newly collected samples, respectively. In patients with TPS ≥1%, OS HRs were 0.74 (95% CI: 0.59, 0.93) and 0.59 (95% CI: 0.48, 0.73) for archival and newly collected samples, respectively. In TPS ≥50%, PFS HRs were similar across archival [0.63 (95% CI: 0.45, 0.89)] and newly collected samples [0.53 (95% CI: 0.38, 0.72)]. In patients with TPS ≥1%, PFS HRs were similar across archival [0.82 (95% CI: 0.66, 1.02)] and newly collected samples [0.83 (95% CI: 0.68, 1.02)].
Conclusion: Pembrolizumab continued to improve OS over docetaxel in intention to treat population and in subsets of patients with newly collected and archival samples.
Trial registration: ClinicalTrials.gov: NCT01905657.
Keywords: PD-L1 expression; pembrolizumab; tumor samples.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Improving our knowledge in PD-L1 testing in lung cancer: the archival sample is 'promoted'!Ann Oncol. 2019 Feb 1;30(2):165-167. doi: 10.1093/annonc/mdy556. Ann Oncol. 2019. PMID: 30715154 No abstract available.
References
-
- Dako. PD-L1 IHC 22C3 pharmDx [package insert], Carpinteria, CA; 2015.
-
- Dolled-Filhart M, Roach C, Toland G. et al. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med 2016; 140(11): 1243–1249. - PubMed
-
- Herbst RS, Baas P, Kim D-W. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540–1550. - PubMed
-
- Cho JH, Sorensen SF, Choi YL. et al. Programmed death ligand 1 expression in paired non-small cell lung cancer tumor samples. Clin Lung Cancer 2017; 18(6): e473–e479. - PubMed
-
- Illidge TL-BG, Cheadle E, Honeychurch J. et al. Radiation therapy induces an adaptive upregulation of PD-L1 on tumor cells which may limit the efficacy of the anti-tumor immune response but can be circumvented by anti-PD-L1. Int J Radiat Oncol Biol Phys 2014; 90(1): S776–3439. [Abstract]
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

