Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms
- PMID: 30657883
- PMCID: PMC6534496
- DOI: 10.1210/er.2018-00160
Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
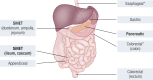
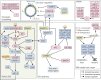
Gastroenteropancreatic (GEP) neuroendocrine neoplasms (NENs) are heterogeneous regarding site of origin, biological behavior, and malignant potential. There has been a rapid increase in data publication during the last 10 years, mainly driven by high-throughput studies on pancreatic and small intestinal neuroendocrine tumors (NETs). This review summarizes the present knowledge on genetic and epigenetic alterations. We integrated the available information from each compartment to give a pathway-based overview. This provided a summary of the critical alterations sustaining neoplastic cells. It also highlighted similarities and differences across anatomical locations and points that need further investigation. GEP-NENs include well-differentiated NETs and poorly differentiated neuroendocrine carcinomas (NECs). NENs are graded as G1, G2, or G3 based on mitotic count and/or Ki-67 labeling index, NECs are G3 by definition. The distinction between NETs and NECs is also linked to their genetic background, as TP53 and RB1 inactivation in NECs set them apart from NETs. A large number of genetic and epigenetic alterations have been reported. Recurrent changes have been traced back to a reduced number of core pathways, including DNA damage repair, cell cycle regulation, and phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling. In pancreatic tumors, chromatin remodeling/histone methylation and telomere alteration are also affected. However, also owing to the paucity of disease models, further research is necessary to fully integrate and functionalize data on deregulated pathways to recapitulate the large heterogeneity of behaviors displayed by these tumors. This is expected to impact diagnostics, prognostic stratification, and planning of personalized therapy.
Copyright © 2019 Endocrine Society.
Figures



References
-
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. - PubMed
-
- Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork . Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21(3):R153–R163. - PubMed
-
- Oberndorfer S. Karzinoide Tumoren des Dünndarms. Frankf Z Pathol. 1907;1:425–432.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

