Splicing events in the control of genome integrity: role of SLU7 and truncated SRSF3 proteins
- PMID: 30657957
- PMCID: PMC6468163
- DOI: 10.1093/nar/gkz014
Splicing events in the control of genome integrity: role of SLU7 and truncated SRSF3 proteins
Abstract
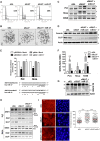
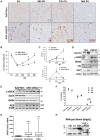
Genome instability is related to disease development and carcinogenesis. DNA lesions are caused by genotoxic compounds but also by the dysregulation of fundamental processes like transcription, DNA replication and mitosis. Recent evidence indicates that impaired expression of RNA-binding proteins results in mitotic aberrations and the formation of transcription-associated RNA-DNA hybrids (R-loops), events strongly associated with DNA injury. We identify the splicing regulator SLU7 as a key mediator of genome stability. SLU7 knockdown results in R-loops formation, DNA damage, cell-cycle arrest and severe mitotic derangements with loss of sister chromatid cohesion (SCC). We define a molecular pathway through which SLU7 keeps in check the generation of truncated forms of the splicing factor SRSF3 (SRp20) (SRSF3-TR). Behaving as dominant negative, or by gain-of-function, SRSF3-TR impair the correct splicing and expression of the splicing regulator SRSF1 (ASF/SF2) and the crucial SCC protein sororin. This unique function of SLU7 was found in cancer cells of different tissue origin and also in the normal mouse liver, demonstrating a conserved and fundamental role of SLU7 in the preservation of genome integrity. Therefore, the dowregulation of SLU7 and the alterations of this pathway that we observe in the cirrhotic liver could be involved in the process of hepatocarcinogenesis.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures







Similar articles
-
Oncogenic SRSF3 in health and diseases.Int J Biol Sci. 2023 Jun 12;19(10):3057-3076. doi: 10.7150/ijbs.83368. eCollection 2023. Int J Biol Sci. 2023. PMID: 37416784 Free PMC article. Review.
-
Splicing regulator SLU7 preserves survival of hepatocellular carcinoma cells and other solid tumors via oncogenic miR-17-92 cluster expression.Oncogene. 2016 Sep 8;35(36):4719-29. doi: 10.1038/onc.2015.517. Epub 2016 Jan 25. Oncogene. 2016. PMID: 26804174
-
Hepatic Knockdown of Splicing Regulator Slu7 Ameliorates Inflammation and Attenuates Liver Injury in Ethanol-Fed Mice.Am J Pathol. 2018 Aug;188(8):1807-1819. doi: 10.1016/j.ajpath.2018.05.004. Epub 2018 Jun 2. Am J Pathol. 2018. PMID: 29870742 Free PMC article.
-
Splicing regulator SLU7 is essential for maintaining liver homeostasis.J Clin Invest. 2014 Jul;124(7):2909-20. doi: 10.1172/JCI74382. Epub 2014 May 27. J Clin Invest. 2014. PMID: 24865429 Free PMC article.
-
SLU7: A New Hub of Gene Expression Regulation-From Epigenetics to Protein Stability in Health and Disease.Int J Mol Sci. 2022 Nov 2;23(21):13411. doi: 10.3390/ijms232113411. Int J Mol Sci. 2022. PMID: 36362191 Free PMC article. Review.
Cited by
-
The Interplay of Cohesin and RNA Processing Factors: The Impact of Their Alterations on Genome Stability.Int J Mol Sci. 2022 Apr 1;23(7):3939. doi: 10.3390/ijms23073939. Int J Mol Sci. 2022. PMID: 35409298 Free PMC article. Review.
-
Compromised nonsense-mediated RNA decay results in truncated RNA-binding protein production upon DUX4 expression.Cell Rep. 2023 Jun 27;42(6):112642. doi: 10.1016/j.celrep.2023.112642. Epub 2023 Jun 13. Cell Rep. 2023. PMID: 37314931 Free PMC article.
-
Oncogenic SRSF3 in health and diseases.Int J Biol Sci. 2023 Jun 12;19(10):3057-3076. doi: 10.7150/ijbs.83368. eCollection 2023. Int J Biol Sci. 2023. PMID: 37416784 Free PMC article. Review.
-
OTUD5 limits replication fork instability by organizing chromatin remodelers.Nucleic Acids Res. 2023 Oct 27;51(19):10467-10483. doi: 10.1093/nar/gkad732. Nucleic Acids Res. 2023. PMID: 37713620 Free PMC article.
-
SRSF3/AMOTL1 splicing axis promotes the tumorigenesis of nasopharyngeal carcinoma through regulating the nucleus translocation of YAP1.Cell Death Dis. 2023 Aug 9;14(8):511. doi: 10.1038/s41419-023-06034-1. Cell Death Dis. 2023. PMID: 37558679 Free PMC article.
References
-
- Aguilera A., Gómez-González B.. Genome instability: a mechanistic view of its causes and consequences. Nat. Rev. Genet. 2008; 9:204–217. - PubMed
-
- Hanahan D., Weinberg R.A.. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674. - PubMed
-
- Gaillard H., García-Muse T., Aguilera A.. Replication stress and cancer. Nat. Rev. Cancer. 2015; 15:276–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

