RecFOR epistasis group: RecF and RecO have distinct localizations and functions in Escherichia coli
- PMID: 30657965
- PMCID: PMC6451095
- DOI: 10.1093/nar/gkz003
RecFOR epistasis group: RecF and RecO have distinct localizations and functions in Escherichia coli
Abstract
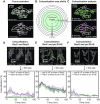
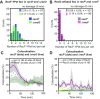
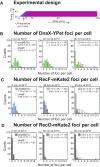
In bacteria, genetic recombination is a major mechanism for DNA repair. The RecF, RecO and RecR proteins are proposed to initiate recombination by loading the RecA recombinase onto DNA. However, the biophysical mechanisms underlying this process remain poorly understood. Here, we used genetics and single-molecule fluorescence microscopy to investigate whether RecF and RecO function together, or separately, in live Escherichia coli cells. We identified conditions in which RecF and RecO functions are genetically separable. Single-molecule imaging revealed key differences in the spatiotemporal behaviours of RecF and RecO. RecF foci frequently colocalize with replisome markers. In response to DNA damage, colocalization increases and RecF dimerizes. The majority of RecF foci are dependent on RecR. Conversely, RecO foci occur infrequently, rarely colocalize with replisomes or RecF and are largely independent of RecR. In response to DNA damage, RecO foci appeared to spatially redistribute, occupying a region close to the cell membrane. These observations indicate that RecF and RecO have distinct functions in the DNA damage response. The observed localization of RecF to the replisome supports the notion that RecF helps to maintain active DNA replication in cells carrying DNA damage.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Fuchs R.P. Tolerance of lesions in E. coli: chronological competition between translesion synthesis and damage avoidance. DNA Repair. 2016; 44:51–58. - PubMed
-
- Cox M.M., Goodman M.F., Kreuzer K.N., Sherratt D.J., Sandler S.J., Marians K.J.. The importance of repairing stalled replication forks. Nature. 2000; 404:37–41. - PubMed
-
- Clark A.J., Sandler S.J.. Homologous genetic recombination: the pieces begin to fall into place. Crit. Rev. Microbiol. 1994; 20:125–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

