Dose Effects of Ammonium Perfluorooctanoate on Lipoprotein Metabolism in APOE*3-Leiden.CETP Mice
- PMID: 30657992
- PMCID: PMC6432869
- DOI: 10.1093/toxsci/kfz015
Dose Effects of Ammonium Perfluorooctanoate on Lipoprotein Metabolism in APOE*3-Leiden.CETP Mice
Abstract
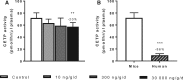
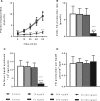
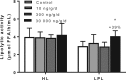
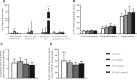
Epidemiological studies have reported positive associations between serum perfluorooctanoic acid (PFOA) and total and non-high-density lipoprotein cholesterol (non-HDL-C) although the magnitude of effect of PFOA on cholesterol lacks consistency. The objectives of this study were to evaluate the effect of PFOA on plasma cholesterol and triglyceride metabolism at various plasma PFOA concentrations relevant to humans, and to elucidate the mechanisms using APOE*3-Leiden.CETP mice, a model with a human-like lipoprotein metabolism. APOE*3-Leiden.CETP mice were fed a Western-type diet with PFOA (10, 300, 30 000 ng/g/d) for 4-6 weeks. PFOA exposure did not alter plasma lipids in the 10 and 300 ng/g/d dietary PFOA dose groups. At 30 000 ng/g/d, PFOA decreased plasma triglycerides (TG), total cholesterol (TC), and non-HDL-C, whereas HDL-C was increased. The plasma lipid alterations could be explained by decreased very low-density lipoprotein (VLDL) production and increased VLDL clearance by the liver through increased lipoprotein lipase activity. The concomitant increase in HDL-C was mediated by decreased cholesteryl ester transfer activity and changes in gene expression of proteins involved in HDL metabolism. Hepatic gene expression and pathway analysis confirmed the changes in lipoprotein metabolism that were mediated for a major part through activation of the peroxisome proliferator-activated receptor (PPAR)α. Our data confirmed the findings from a phase 1 clinical trial in humans that demonstrated high serum or plasma PFOA levels resulted in lower cholesterol levels. The study findings do not show an increase in cholesterol at environmental or occupational levels of PFOA exposure, thereby indicating these findings are associative rather than causal.
Keywords: cholesterol; lipoproteins; non-HDL cholesterol; perfluorooctanoic acid (PFOA); peroxisome proliferator-activated receptor (PPAR)α; triglycerides.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures



References
-
- Bijland S., Pieterman E. J., Maas A. C., van der Hoorn J. W., van Erk M. J., van Klinken J. B., Havekes L. M., van Dijk K. W., Princen H. M., Rensen P. C. (2010). Fenofibrate increases very low density lipoprotein triglyceride production despite reducing plasma triglyceride levels in APOE*3-Leiden.CETP mice. J. Biol. Chem. 285, 25168–25175. - PMC - PubMed
-
- Bijland S., Rensen P. C., Pieterman E. J., Maas A. C., van der Hoorn J. W., van Erk M. J., Havekes L. M., Willems van Dijk K., Chang S. C., Ehresman D. J., et al. (2011). Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol. Sci. 123, 290–303. - PubMed
-
- Bjork J. A., Butenhoff J. L., Wallace K. B. (2011). Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 288, 8–17. - PubMed
-
- Bougarne N., Weyers B., Desmet S. J., Deckers J., Ray D. W., Staels B., De Bosscher K. (2018). Molecular actions of PPARalpha in lipid metabolism and inflammation. Endocr. Rev. 39, 760–802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

