Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery
- PMID: 30658124
- PMCID: PMC6379151
- DOI: 10.1016/j.jconrel.2019.01.013
Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery
Abstract
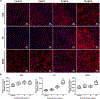
Peripheral nerve injury accounts for roughly 2.8% of all trauma patients with an annual cost of 7 billion USD in the U.S. alone. Current treatment options rely on surgical intervention with the use of an autograft, despite associated shortcomings. Engineered nerve guidance conduits, stem cell therapies, and transient electrical stimulation have reported to increase speeds of functional recovery. As an alternative to the conduction effects of electrical stimulation, we have designed and optimized a nerve guidance conduit with aligned microchannels for the sustained release of a small molecule drug that promotes nerve impulse conduction. A biodegradable chitosan structure reinforced with drug-loaded halloysite nanotubes (HNT) was formed into a foam-like conduit with interconnected, longitudinally-aligned pores with an average pore size of 59.3 ± 14.2 μm. The aligned composite with HNTs produced anisotropic mechanical behavior with a Young's modulus of 0.33 ± 0.1 MPa, very similar to that of native peripheral nerve. This manuscript reports on the sustained delivery of 4-Aminopyridine (4AP, molecular weight 94.1146 g/mol), a potassium-channel blocker as a growth factor alternative to enhance the rate of nerve regeneration. The conduit formulation released a total of 30 ± 2% of the encapsulated 4AP in the first 7 days. Human Schwann cells showed elevated expression of key proteins such as nerve growth factor, myelin protein zero, and brain derived neurotrophic factor in a 4AP dose dependent manner. Preliminary in vivo studies in a critical-sized sciatic nerve defect in Wistar rats confirmed conduit suturability and strength to withstand ambulatory forces over 4 weeks of their implantation. Histological evaluations suggest conduit biocompatibility and Schwann cell infiltration and organization within the conduit and lumen. These nerve guidance conduits and 4AP sustained delivery may serve as an attractive strategy for nerve repair and regeneration.
Keywords: Halloysite nanotube; Nerve guidance conduit; Peripheral nerve regeneration; Polymer composite; Sciatic nerve defect; Small molecule drug delivery; Sustained release.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
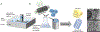







References
-
- Burnett MG and Zager EL, “Pathophysiology of peripheral nerve injury: a brief review,” Neurosurgical focus, vol. 16, pp. 1–7, 2004. - PubMed
-
- Gu X, Ding F, and Williams DF, “Neural tissue engineering options for peripheral nerve regeneration,” Biomaterials, vol. 35, pp. 6143–6156, 2014. - PubMed
-
- Chen P, Piao X, and Bonaldo P, “Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury,” Acta neuropathologica, vol. 130, pp. 605–618, 2015. - PubMed
-
- Griffin JW, Hogan MV, Chhabra AB, and Deal DN, “Peripheral nerve repair and reconstruction,” The Journal of Bone & Joint Surgery, vol. 95, pp. 2144–2151, 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

