Long-term RNAi knockdown of α-synuclein in the adult rat substantia nigra without neurodegeneration
- PMID: 30658149
- PMCID: PMC6440542
- DOI: 10.1016/j.nbd.2019.01.004
Long-term RNAi knockdown of α-synuclein in the adult rat substantia nigra without neurodegeneration
Abstract
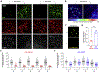
α-Synuclein plays a central role in the pathogenesis of Parkinson's disease (PD); interventions that decrease its expression appear neuroprotective in PD models. Successful translation of these observations into effective therapies will be dependent on the safety of suppressing α-synuclein expression in the adult brain. We investigated long-term α-synuclein knockdown in the adult rat CNS. 8-month old animals received either AAV-sh[Snca] (an RNA interference vector targeting the Snca mRNA transcript) or AAV-sh[Ctrl] (a control vector) unilaterally into the substantia nigra. No signs of systemic toxicity or motor dysfunction were observed in either experimental group over 12 months. Viral transgene expression persisted to 12 months post-inoculation, at which point Snca mRNA expression in substantia nigra dopaminergic neurons of animals that received AAV-sh[Snca] was decreased by ≈90%, and α-synuclein immunoreactivity by >70% relative to the control side. Stereological quantification of Nissl-labeled neurons showed no evidence of neurodegeneration in the substantia nigra 12 months after inoculation with either vector, and we observed abundant dopaminergic neurons with minimal α-synuclein immunoreactivity that appeared otherwise unremarkable in the AAV-sh[Snca] group. Despite the absence of neurodegeneration, some loss of TH expression was evident in nigral neurons after transduction with either vector, presumably a non-specific consequence of vector delivery, cellular transduction, or expression of shRNA or GFP. We conclude that long-term α-synuclein knockdown in the substantia nigra does not cause significant functional deficits in the ascending dopaminergic projection, or neurodegeneration. These findings are encouraging that it may be feasible to target α-synuclein expression therapeutically in PD.
Published by Elsevier Inc.
Figures
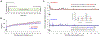

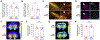
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

