Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study
- PMID: 30658418
- PMCID: PMC6466225
- DOI: 10.3390/bioengineering6010008
Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study
Abstract
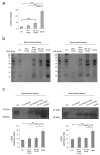
Extracellular vesicles (EV) are small membrane structures released by cells that act as potent mediators of intercellular communication. The study of EV biology is important, not only to strengthen our knowledge of their physiological roles, but also to better understand their involvement in several diseases. In the field of biomedicine they have been studied as a novel source of biomarkers and drug delivery vehicles. The most commonly used method for EV enrichment in crude pellet involves serial centrifugation and ultracentrifugation. Recently, different protocols and techniques have been developed to isolate EV that imply less time and greater purification. Here we carry out a comparative analysis of three methods to enrich EV from plasma of healthy controls: ultracentrifugation, ExoQuickTM precipitation solution (System Biosciences), and Total Exosome Isolation kit (Invitrogen). Our results show that commercial precipitation reagents are more efficient and enable higher EV enrichment factors compared with traditional ultracentrifugation, although subsequent imaging analysis is not possible with some of them. We hope that this work will contribute to the current research on isolation techniques to assist the progress of clinical applications with diagnostic or therapeutic objectives.
Keywords: enrichment; extracellular vesicles; lateral flow immunoassay; nanoparticle tracking analysis; ultracentrifugation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

