Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D₃
- PMID: 30658432
- PMCID: PMC6358963
- DOI: 10.3390/ijms20020385
Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D₃
Abstract
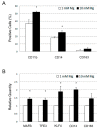
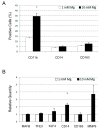
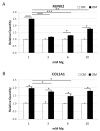
Magnesium (Mg) is crucial for bone health. Low concentrations of Mg inhibit the activity of osteoblasts while promoting that of osteoclasts, with the final result of inducing osteopenia. Conversely, little is known about the effects of high concentrations of extracellular Mg on osteoclasts and osteoblasts. Since the differentiation and activation of these cells is coordinated by vitamin D₃ (VD3), we investigated the effects of high extracellular Mg, as well as its impact on VD3 activity, in these cells. U937 cells were induced to osteoclastic differentiation by VD3 in the presence of supra-physiological concentrations (>1 mM) of extracellular Mg. The effect of high Mg concentrations was also studied in human bone-marrow-derived mesenchymal stem cells (bMSCs) induced to differentiate into osteoblasts by VD3. We demonstrate that high extra-cellular Mg levels potentiate VD3-induced osteoclastic differentiation, while decreasing osteoblastogenesis. We hypothesize that Mg might reprogram VD3 activity on bone remodeling, causing an unbalanced activation of osteoclasts and osteoblasts.
Keywords: biodegradable magnesium alloys; hematopoietic U937 cells; human bone-marrow mesenchymal stem cells; magnesium; osteoclasts; vitamin D3.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Magnesium favors the capacity of vitamin D3 to induce the monocyte differentiation of U937 cells.Magnes Res. 2021 Aug 1;34(3):114-129. doi: 10.1684/mrh.2021.0490. Magnes Res. 2021. PMID: 34859787
-
Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis.J Pineal Res. 2018 Apr;64(3):10.1111/jpi.12465. doi: 10.1111/jpi.12465. Epub 2018 Jan 17. J Pineal Res. 2018. PMID: 29285799 Free PMC article.
-
Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture.Acta Biomater. 2015 Nov;27:294-304. doi: 10.1016/j.actbio.2015.08.042. Epub 2015 Aug 28. Acta Biomater. 2015. PMID: 26318802
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Mechanism of inhibitory action of eldecalcitol, an active vitamin D analog, on bone resorption in vivo.J Steroid Biochem Mol Biol. 2013 Jul;136:171-4. doi: 10.1016/j.jsbmb.2012.11.010. Epub 2012 Dec 5. J Steroid Biochem Mol Biol. 2013. PMID: 23220095 Review.
Cited by
-
Analysis of Intracellular Magnesium and Mineral Depositions during Osteogenic Commitment of 3D Cultured Saos2 Cells.Int J Mol Sci. 2020 Mar 30;21(7):2368. doi: 10.3390/ijms21072368. Int J Mol Sci. 2020. PMID: 32235449 Free PMC article.
-
From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights.Int J Mol Sci. 2019 Oct 4;20(19):4925. doi: 10.3390/ijms20194925. Int J Mol Sci. 2019. PMID: 31590328 Free PMC article. Review.
-
Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate.Membranes (Basel). 2023 Jul 25;13(8):691. doi: 10.3390/membranes13080691. Membranes (Basel). 2023. PMID: 37623752 Free PMC article.
-
Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications.Nanomaterials (Basel). 2024 Feb 28;14(5):441. doi: 10.3390/nano14050441. Nanomaterials (Basel). 2024. PMID: 38470772 Free PMC article.
-
Antibacterial peptides inhibit MC3T3-E1 cells apoptosis induced by TNF-α through p38 MAPK pathway.Ann Transl Med. 2020 Aug;8(15):943. doi: 10.21037/atm-20-5338. Ann Transl Med. 2020. PMID: 32953743 Free PMC article.
References
-
- Kuby S.A., Noltman E.A. ATP-Creatine Transphosphorylase. The Enzymes. 2nd ed. Academic Press; New York, NY, USA: 1962. pp. 515–603.

