KRIT1 Loss-Of-Function Associated with Cerebral Cavernous Malformation Disease Leads to Enhanced S-Glutathionylation of Distinct Structural and Regulatory Proteins
- PMID: 30658464
- PMCID: PMC6356485
- DOI: 10.3390/antiox8010027
KRIT1 Loss-Of-Function Associated with Cerebral Cavernous Malformation Disease Leads to Enhanced S-Glutathionylation of Distinct Structural and Regulatory Proteins
Abstract
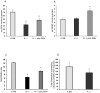
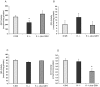
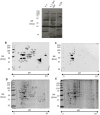
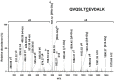
Loss-of-function mutations in the KRIT1 gene are associated with the pathogenesis of cerebral cavernous malformations (CCMs), a major cerebrovascular disease still awaiting therapies. Accumulating evidence demonstrates that KRIT1 plays an important role in major redox-sensitive mechanisms, including transcriptional pathways and autophagy, which play major roles in cellular homeostasis and defense against oxidative stress, raising the possibility that KRIT1 loss has pleiotropic effects on multiple redox-sensitive systems. Using previously established cellular models, we found that KRIT1 loss-of-function affects the glutathione (GSH) redox system, causing a significant decrease in total GSH levels and increase in oxidized glutathione disulfide (GSSG), with a consequent deficit in the GSH/GSSG redox ratio and GSH-mediated antioxidant capacity. Redox proteomic analyses showed that these effects are associated with increased S-glutathionylation of distinct proteins involved in adaptive responses to oxidative stress, including redox-sensitive chaperonins, metabolic enzymes, and cytoskeletal proteins, suggesting a novel molecular signature of KRIT1 loss-of-function. Besides providing further insights into the emerging pleiotropic functions of KRIT1, these findings point definitively to KRIT1 as a major player in redox biology, shedding new light on the mechanistic relationship between KRIT1 loss-of-function and enhanced cell sensitivity to oxidative stress, which may eventually lead to cellular dysfunctions and CCM disease pathogenesis.
Keywords: KRIT1; altered redox homeostasis and signaling; cerebral cavernous malformations; mass spectrometry; oxidative post-translational modifications; oxidative stress; protein S-glutathionylation; redox proteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease.Free Radic Biol Med. 2018 Feb 1;115:202-218. doi: 10.1016/j.freeradbiomed.2017.11.014. Epub 2017 Nov 21. Free Radic Biol Med. 2018. PMID: 29170092 Free PMC article.
-
KRIT1: A Traffic Warden at the Busy Crossroads Between Redox Signaling and the Pathogenesis of Cerebral Cavernous Malformation Disease.Antioxid Redox Signal. 2023 Mar;38(7-9):496-528. doi: 10.1089/ars.2021.0263. Epub 2022 Nov 1. Antioxid Redox Signal. 2023. PMID: 36047808 Free PMC article. Review.
-
Production of KRIT1-knockout and KRIT1-knockin Mouse Embryonic Fibroblasts as Cellular Models of CCM Disease.Methods Mol Biol. 2020;2152:151-167. doi: 10.1007/978-1-0716-0640-7_12. Methods Mol Biol. 2020. PMID: 32524551
-
Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: Two sides of the same coin.Int J Biochem Cell Biol. 2016 Dec;81(Pt B):254-270. doi: 10.1016/j.biocel.2016.09.011. Epub 2016 Sep 14. Int J Biochem Cell Biol. 2016. PMID: 27639680 Free PMC article. Review.
-
KRIT1 Deficiency Promotes Aortic Endothelial Dysfunction.Int J Mol Sci. 2019 Oct 5;20(19):4930. doi: 10.3390/ijms20194930. Int J Mol Sci. 2019. PMID: 31590384 Free PMC article.
Cited by
-
Side Effects of Curcumin: Epigenetic and Antiproliferative Implications for Normal Dermal Fibroblast and Breast Cancer Cells.Antioxidants (Basel). 2019 Sep 9;8(9):382. doi: 10.3390/antiox8090382. Antioxidants (Basel). 2019. PMID: 31505772 Free PMC article.
-
Vitamin D Deficiency and the Risk of Cerebrovascular Disease.Antioxidants (Basel). 2020 Apr 17;9(4):327. doi: 10.3390/antiox9040327. Antioxidants (Basel). 2020. PMID: 32316584 Free PMC article. Review.
-
Effect of Pholiota nameko Polysaccharides Inhibiting Methylglyoxal-Induced Glycation Damage In Vitro.Antioxidants (Basel). 2021 Oct 10;10(10):1589. doi: 10.3390/antiox10101589. Antioxidants (Basel). 2021. PMID: 34679724 Free PMC article.
-
Heterozygous Loss of KRIT1 in Mice Affects Metabolic Functions of the Liver, Promoting Hepatic Oxidative and Glycative Stress.Int J Mol Sci. 2022 Sep 22;23(19):11151. doi: 10.3390/ijms231911151. Int J Mol Sci. 2022. PMID: 36232456 Free PMC article.
-
Next-Generation Sequencing Advances the Genetic Diagnosis of Cerebral Cavernous Malformation (CCM).Antioxidants (Basel). 2022 Jun 29;11(7):1294. doi: 10.3390/antiox11071294. Antioxidants (Basel). 2022. PMID: 35883785 Free PMC article.
References
-
- Trapani E., Retta S.F. Cerebral cavernous malformation (CCM) disease: From monogenic forms to genetic susceptibility factors. J. Neurosurg. Sci. 2015;59:201–209. - PubMed
-
- Fontanella M., Bacigaluppi S. Treatment of cerebral cavernous malformations: Where do we stand. J. Neurosurg. Sci. 2015;59:199–200. - PubMed

