Monitoring the Disulfide Bonds of Folding Isomers of Synthetic CTX A3 Polypeptide Using MS-Based Technology
- PMID: 30658470
- PMCID: PMC6356385
- DOI: 10.3390/toxins11010052
Monitoring the Disulfide Bonds of Folding Isomers of Synthetic CTX A3 Polypeptide Using MS-Based Technology
Abstract
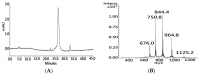
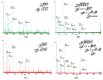
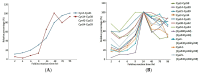
Native disulfide formation is crucial to the process of disulfide-rich protein folding in vitro. As such, analysis of the disulfide bonds can be used to track the process of the folding reaction; however, the diverse structural isomers interfere with characterization due to the non-native disulfide linkages. Previously, a mass spectrometry (MS) based platform coupled with peptide demethylation and an automatic disulfide bond searching engine demonstrated the potential to screen disulfide-linked peptides for the unambiguous assignment of paired cysteine residues of toxin components in cobra venom. The developed MS-based platform was evaluated to analyze the disulfide bonds of structural isomers during the folding reaction of synthetic cardiotoxin A3 polypeptide (syn-CTX A3), an important medical component in cobra venom. Through application of this work flow, a total of 13 disulfide-linked peptides were repeatedly identified across the folding reaction, and two of them were found to contain cysteine pairings, like those found in native CTX A3. Quantitative analysis of these disulfide-linked peptides showed the occurrence of a progressive disulfide rearrangement that generates a native disulfide bond pattern on syn-CTX A3 folded protein. The formation of these syn-CTX A3 folded protein reaches a steady level in the late stage of the folding reaction. Biophysical and cell-based assays showed that the collected syn-CTX A3 folded protein have a β-sheet secondary structure and cytotoxic activity similar to that of native CTX A3. In addition, the immunization of the syn-CTX A3 folded proteins could induce neutralization antibodies against the cytotoxic activity of native CTX A3. In contrast, these structure activities were poorly observed in the other folded isomers with non-native disulfide bonds. The study highlights the ability of the developed MS platform to assay isomers with heterogeneous disulfide bonds, providing insight into the folding mechanism of the bioactive protein generation.
Keywords: cardiotoxin; disulfide bond analysis; folding isomer; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Fully oxidized scrambled isomers are essential and predominant folding intermediates of cardiotoxin-III.FEBS Lett. 2006 Jan 23;580(2):656-60. doi: 10.1016/j.febslet.2005.12.064. Epub 2005 Dec 28. FEBS Lett. 2006. PMID: 16412427
-
Unfolding and refolding of cardiotoxin III elucidated by reversible conversion of the native and scrambled species.Biochemistry. 1998 May 12;37(19):6745-51. doi: 10.1021/bi9714565. Biochemistry. 1998. PMID: 9578558
-
Unfolding/folding studies on cobrotoxin from Taiwan cobra venom: pH and GSH/GSSG govern disulfide isomerization at the C-terminus.Arch Biochem Biophys. 1998 Jun 1;354(1):1-8. doi: 10.1006/abbi.1998.0660. Arch Biochem Biophys. 1998. PMID: 9633591
-
Folding of peptides and proteins: role of disulfide bonds, recent developments.Biomol Concepts. 2013 Dec;4(6):597-604. doi: 10.1515/bmc-2013-0022. Biomol Concepts. 2013. PMID: 25436759 Review.
-
Overview of the regulation of disulfide bond formation in Peptide and protein folding.Curr Protoc Protein Sci. 2014 Apr 1;76:28.6.1-28.6.6. doi: 10.1002/0471140864.ps2806s76. Curr Protoc Protein Sci. 2014. PMID: 24692015 Review.
Cited by
-
Variability in the Spatial Structure of the Central Loop in Cobra Cytotoxins Revealed by X-ray Analysis and Molecular Modeling.Toxins (Basel). 2022 Feb 18;14(2):149. doi: 10.3390/toxins14020149. Toxins (Basel). 2022. PMID: 35202176 Free PMC article.
-
The myth of cobra venom cytotoxin: More than just direct cytolytic actions.Toxicon X. 2022 Apr 4;14:100123. doi: 10.1016/j.toxcx.2022.100123. eCollection 2022 Jun. Toxicon X. 2022. PMID: 35434602 Free PMC article.
-
Synthetic peptides to produce antivenoms against the Cys-rich toxins of arachnids.Toxicon X. 2020 May 5;6:100038. doi: 10.1016/j.toxcx.2020.100038. eCollection 2020 Jun. Toxicon X. 2020. PMID: 32550593 Free PMC article.
-
Development of a Broad-Spectrum Antiserum against Cobra Venoms Using Recombinant Three-Finger Toxins.Toxins (Basel). 2021 Aug 10;13(8):556. doi: 10.3390/toxins13080556. Toxins (Basel). 2021. PMID: 34437427 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

