How to design a dose-finding study using the continual reassessment method
- PMID: 30658575
- PMCID: PMC6339349
- DOI: 10.1186/s12874-018-0638-z
How to design a dose-finding study using the continual reassessment method
Abstract
Introduction: The continual reassessment method (CRM) is a model-based design for phase I trials, which aims to find the maximum tolerated dose (MTD) of a new therapy. The CRM has been shown to be more accurate in targeting the MTD than traditional rule-based approaches such as the 3 + 3 design, which is used in most phase I trials. Furthermore, the CRM has been shown to assign more trial participants at or close to the MTD than the 3 + 3 design. However, the CRM's uptake in clinical research has been incredibly slow, putting trial participants, drug development and patients at risk. Barriers to increasing the use of the CRM have been identified, most notably a lack of knowledge amongst clinicians and statisticians on how to apply new designs in practice. No recent tutorial, guidelines, or recommendations for clinicians on conducting dose-finding studies using the CRM are available. Furthermore, practical resources to support clinicians considering the CRM for their trials are scarce.
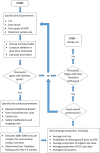
Methods: To help overcome these barriers, we present a structured framework for designing a dose-finding study using the CRM. We give recommendations for key design parameters and advise on conducting pre-trial simulation work to tailor the design to a specific trial. We provide practical tools to support clinicians and statisticians, including software recommendations, and template text and tables that can be edited and inserted into a trial protocol. We also give guidance on how to conduct and report dose-finding studies using the CRM.
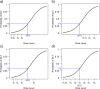
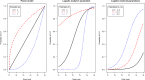
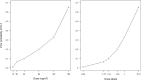
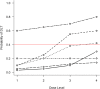
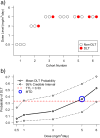
Results: An initial set of design recommendations are provided to kick-start the design process. To complement these and the additional resources, we describe two published dose-finding trials that used the CRM. We discuss their designs, how they were conducted and analysed, and compare them to what would have happened under a 3 + 3 design.
Conclusions: The framework and resources we provide are aimed at clinicians and statisticians new to the CRM design. Provision of key resources in this contemporary guidance paper will hopefully improve the uptake of the CRM in phase I dose-finding trials.
Keywords: Adaptive designs; Continual reassessment method; Dose escalation; Dose-finding; Maximum tolerated dose; Phase I trials.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AB is an employee and shareholder of Roche Products Ltd. KB owns equity in GlaxoSmithKline and AstraZeneca and has received travel and conference fee reimbursements from Merck and Roche. APG is an employee of UCB Pharma Ltd. All other authors have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Babb JS, Rogatko A. Bayesian methods for phase I cancer clinical trials. In: Geller NL, editor. Advances in clinical trial biostatistics. New York, NY: Marcel Dekker; 2004. pp. 1–40.
-
- Carter SK. Study design principles for the clinical evaluation of new drugs as developed by the chemotherapy programme of the National Cancer Institute. In: Staquet MJ, editor. The Design of Clinical Trials in Cancer Therapy. Editions Scientifique Europe. 1973. pp. 242–289.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

