Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: Results from the EMERGE multicenter retrospective chart review study
- PMID: 30658600
- PMCID: PMC6339387
- DOI: 10.1186/s12885-019-5301-5
Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: Results from the EMERGE multicenter retrospective chart review study
Abstract
Background: The "EMERGE" study, aimed to capture real-life management patterns and outcomes in metastatic breast cancer (MBC) in Greece, also accounting for hormone (HR) and human epidermal growth factor receptor 2 (HER2) status.
Methods: "EMERGE" was a multicenter, retrospective cohort study of adult MBC patients diagnosed between 01-Janaury-2010 and 30-June-2012, either de novo or having progressed from a non-metastatic state. Patient data, including treatment patterns and outcomes, were mainly abstracted through medical chart review.
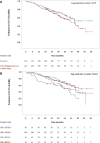
Results: 386 patients were enrolled by 16 hospital-based oncologists between 12-March-2013 and 31-March-2015. The median look-back period was 29.1 months. At MBC diagnosis, 56.1% of the patients were HR+/HER2-, 16.6% HR+/HER2+, 14.5% HR-/HER2-, and 12.8% HR-/HER2+. In the first line setting, chemotherapy, targeted therapy and endocrine therapy were received by 76.7, 52.4, and 28.3% of the overall population, and by 66.5/36.2/42.0%, 80.4/80.4/28.6%, 88.4/90.7/0.0, and 95.6%/56.5/6.5% of the HR+/HER2-, HR+/HER2+, HR-/HER2+, HR-/HER2- subpopulations, respectively. In the overall population, the disease progression incidence rate was 0.57 [95% confidence interval (CI): 0.48-0.67] per person-year; median progression-free survival (PFS) was 22.4 (95% CI: 20.4-24.7) and overall survival (OS) was 45.0 (95% CI: 40.9-55.0) months. Median PFS was 24.6 (95% CI: 21.3-27.9) in HR+/HER2-, 19.7 (95% CI: 12.9-25.9) in HR+/HER2+, 23.0 (95% CI: 16.6-29.7) in HR-/HER2+ and 18.3 (95% CI: 10.0-24.7) months in HR-/HER2- subpopulations. A multivariable Cox proportional hazards model, adjusted among other factors for age and duration of diagnosis, HR and HER2 status, demonstrated that in the overall population PFS was better among those receiving first line endocrine therapy (hazard ratio: 0.70; 95%CI: 0.51-0.95; p = 0.024).
Conclusions: "EMERGE" demonstrates differences between HR/HER2 subtypes in clinical outcomes and divergence from evidence-based guideline recommendations for MBC management, especially as it pertains to the HR+/HER2- patients in which chemotherapy was favored over endocrine therapy in the first line setting.
Study registration: The study has been registered on the electronic Registry of Non-Interventional Studies (RNIS) posted on the website of the Hellenic Association of Pharmaceutical Companies (SFEE): https://www.dilon.sfee.gr/studiesp_d.php?meleti_id=NIS-OGR-XXX-2012/1.
Keywords: Greece; Hormone receptor; Human epidermal growth factor receptor 2; Metastatic breast cancer; Overall survival; Progression-free survival; Treatment patterns.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and all applicable local requirements. The original study protocol was reviewed and approved by the competent institutional review boards of the participating hospital sites before the enrollment of any patient into the study and the performance of any study-related procedure. The IRBs which approved the conduct of this study were those of the: General Hospital of Athens “Alexandra”, Athens, Greece; Prefecture General Hospital for Cancer Treatment “Agioi Anargyri”, Athens, Greece (two principal investigators); University General Hospital of Ioannina, Ioannina, Greece; General University Hospital of Heraklion, Crete, Greece; Regional Hospital for Cancer Treatment “Agios Savvas”, Athens, Greece (two principal investigators); 251 Air Force Hospital, Athens, Greece; “Mitera” Maternity Hospital, Athens, Greece; “IASO” General Hospital, Athens, Greece; General Hospital of Chania “Agios Georgios”, Crete, Greece; Piraeus Regional General Hospital for Cancer Treatment “Metaxa”, Piraeus, Greece; General Hospital of Patras “Ag. Andreas”, Patras, Greece; “Metropolitan” Hospital, Piraeus, Greece; Diagnostic Centre of Athens “Hygeia”, Athens, Greece; and “Attikon” University Hospital, Athens, Greece. All subsequent amendments of the study protocol were also approved by the competent institutional review boards. Due to the retrospective chart review design that included the collection of secondary data only and in order to minimize the risk of biasing the clinical outcomes by exclusion from study participation of deceased subjects or patients not able to provide consent, informed consent requirement was not applied.
Consent for publication
Not applicable.
Competing interests
AK has nothing to disclose. AA has received honoraria for consultancy in advisory boards from Pfizer, Novartis and Roche. ES has received speaker honoraria from Novartis, BMS, AstraZeneca, Genesis, MSD, Amgen, Merck and Roche. GK has received honoraria for consultancy in advisory boards from Novartis, BMS, AstraZeneca, Genesis, MSD and Roche. AP has received honoraria for consultancy in advisory boards from AstraZeneca, Novartis and Roche and research grants from BMS. CP has received speaker honoraria and honoraria for consultancy in advisory boards from Novartis, AstraZeneca, Genesis, MSD, Amgen, Merck and Roche and research grants from BMS and Roche.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- Surveillance Epidemiology and End Results. SEER Stat Fact Sheets: Breast Cancer. SEER Cancer Statistics Review 2006–2012. Available at https://seer.cancer.gov/statfacts/html/breast.html. Accessed December 6, 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

