Intramuscular injection, intravenous infusion, and intravenous bolus of oxytocin in the third stage of labor for prevention of postpartum hemorrhage: a three-arm randomized control trial
- PMID: 30658605
- PMCID: PMC6339323
- DOI: 10.1186/s12884-019-2181-2
Intramuscular injection, intravenous infusion, and intravenous bolus of oxytocin in the third stage of labor for prevention of postpartum hemorrhage: a three-arm randomized control trial
Abstract
Background: Oxytocin for postpartum hemorrhage (PPH) prophylaxis is commonly administered by either intramuscular (IM) injection or intravenous (IV) infusion with both routes recommended equally and little discussion of potential differences between the two. This trial assesses the effectiveness and safety of 10 IU oxytocin administered as IM injection versus IV infusion and IV bolus during the third stage of labor for PPH prophylaxis.
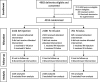
Methods: In two tertiary level Egyptian maternity hospitals, women delivering vaginally without exposure to pre-delivery uterotonics were randomized to one of three prophylactic oxytocin administration groups after delivery of the baby. Blood loss was measured 1 h after delivery, and side effects were recorded. Primary outcomes were mean postpartum blood loss and proportion of women with postpartum blood loss ≥500 ml in this open-label, three-arm, parallel, randomized controlled trial.
Results: Four thousand nine hundred thirteen eligible, consenting women were randomized. Compared to IM injection, mean blood loss was 5.9% less in the IV infusion arm (95% CI: -8.5, - 3.3) and 11.1% less in the IV bolus arm (95% CI: -14.7, - 7.8). Risk of postpartum blood loss ≥500 ml in the IV infusion arm was significantly less compared to IM injection (0.8% vs. 1.5%, RR = 0.50, 95% CI: 0.27, 0.91). No side effects were reported in any arm.
Conclusions: Intravenous oxytocin is more effective than intramuscular injection for the prevention of PPH in the third stage of labor. Oxytocin delivered by IV bolus presents no safety concerns after vaginal delivery and should be considered a safe option for PPH prophylaxis.
Trial registration: clinicaltrials.gov # NCT01914419 , posted August 2, 2013.
Keywords: Bolus oxytocin; Intramuscular oxytocin; Intravenous oxytocin; Oxytocic; Oxytocin; Oxytocin prophylaxis; Postpartum blood loss; Postpartum hemorrhage; Route of administration; Third stage of labor.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved on July 3, 2013 by the Ethics Committee of the Faculty of Medicine at Alexandria University and September 1, 2013 by the Ethics Committee of El Galaa Teaching Hospital. Written consent was obtained from all participants upon arrival to the labor ward.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- WHO . WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: Dept. of reproductive health and research, WHO; 2012. pp. 1–41. - PubMed
-
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013;10(CD001808):i–90. - PubMed
-
- NICE. Intrapartum care: Care of healthy women and their babies during childbirth, Guideline 190. 2014 February [cited 2017 19 April ]; Available from: https://www.nice.org.uk/guidance/cg190/chapter/Recommendations.
-
- ICM-FIGO. Joint statement: management of the third stage of labour to prevent post-partum haemorrhage. J Midwifery Womens Health. 2004;49(1):76–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical