The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial
- PMID: 30658629
- PMCID: PMC6339353
- DOI: 10.1186/s12955-019-1090-4
The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial
Abstract
Background: This study evaluated the impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy.
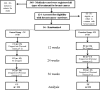
Methods: Older breast cancer survivors were randomized into two groups: combined training: resistance + aerobic exercise program for nine months (n = 18) or control group (n = 18). Quality of life was assessed by the questionnaires SF36, EORTC QLQ-C30, and EORTC QLQ-BR23 at baseline, and at three, six, and nine months. The exercise group performed 40 min of resistance exercises on machines followed by 30 min of aerobic training on a treadmill 3x/wk. Repeated measures ANOVA was used to compare the groups over time.
Results: Significant time x group interactions and moderate to high effect sizes were found for physical functioning, physical health, bodily pain, general health perception, vitality, social functioning, fatigue, sleep disturbance, body image, and upset by hair loss, favoring the exercise group.
Conclusion: This study demonstrated the potential benefits and high clinical relevance of exercise programs to improve quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy.
Keywords: Breast cancer; Combined exercise; Hormone therapy; Menopause; Physical activity; Public health.
Conflict of interest statement
Ethics approval and consent to participate
The study received approval from the Committee on Ethics in Research of the State University of Sao Paulo, UNESP, protocol number: 6727715.1.0000.5402 / 2015. Participants were informed about the objectives of the study and written informed consent was obtained.
Consent for publication
No individual’s personal data is included.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee MC, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw KCA, Sullivan M, Takeda F. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. - DOI - PubMed
-
- Arem H, Sorkin M, Cartmel B, Fiellin M, Capozza S, Harrigan M, Ercolano E, Zhou Y, Sanft T, Gross C, Schmitz K, Neogi T, Hershman D, Ligibel J, Irwin ML. Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: the hormones and physical exercise (HOPE) study. J Cancer Surviv. 2016;10(4):654–662. doi: 10.1007/s11764-015-0511-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical