Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue
- PMID: 30658634
- PMCID: PMC6339284
- DOI: 10.1186/s12944-019-0963-1
Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue
Abstract
Background: Prenatal dexamethasone treatment has been shown to enhance the susceptibility of offspring to postnatal high-fat (HF) diet-induced programmed obesity. We investigated the metabolic phenotypes, nutrient-sensing signal and circadian-clock genes in adipose tissue that are programmed by prenatal dexamethasone exposure and postnatal HF diet.
Methods: Male offspring of Sprague-Dawley rats were divided into four experimental groups: normal diet, prenatal dexamethasone exposure, postnatal HF diet, and prenatal dexamethasone plus postnatal HF diet. Postnatal HF diet was prescribed from weaning to 6 months of age.
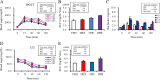
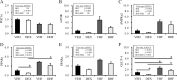
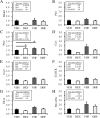
Results: Prenatal dexamethasone and postnatal HF diet exerted synergistic effects on body weight and visceral adiposity, whereas prenatal dexamethasone and postnatal HF diet altered the metabolic profile and caused leptin dysregulation. Prenatal dexamethasone and postnatal HF diet distinctly influenced nutrient-sensing molecules and circadian-clock genes in adipose tissue. The mRNA expression of mTOR, AMPK-α2, PPAR-α, and PPAR-γ was suppressed by prenatal dexamethasone but enhanced by postnatal HF diet.
Conclusion: Prenatal dexamethasone and postnatal HF treatment cause dysregulation of nutrient-sensing molecules and circadian-clock genes in visceral adipose tissue. Characterizing altered nutrient-sensing molecules and circadian-clock genes has potential therapeutic relevance with respect to the pathogenesis and treatment of prenatal stress and postnatal HF diet-related metabolic disorders.
Keywords: Adipose tissue; Circadian-clock; Nutrition sensory signals; Postnatal high-fat diet; Prenatal dexamethasone.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was approved by the Institutional Animal Care and Use Committee of the Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Prenatal dexamethasone and postnatal high-fat diet have a synergistic effect of elevating blood pressure through a distinct programming mechanism of systemic and adipose renin-angiotensin systems.Lipids Health Dis. 2018 Mar 14;17(1):50. doi: 10.1186/s12944-018-0701-0. Lipids Health Dis. 2018. PMID: 29540174 Free PMC article.
-
High-fat/fructose feeding during prenatal and postnatal development in female rats increases susceptibility to renal and metabolic injury later in life.Am J Physiol Regul Integr Comp Physiol. 2013 Feb 15;304(4):R278-85. doi: 10.1152/ajpregu.00433.2012. Epub 2012 Dec 19. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23255587 Free PMC article.
-
Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats.Int J Mol Sci. 2016 Sep 22;17(10):1610. doi: 10.3390/ijms17101610. Int J Mol Sci. 2016. PMID: 27669212 Free PMC article.
-
Fetal programming of adipose tissue: effects of intrauterine growth restriction and maternal obesity/high-fat diet.Semin Reprod Med. 2011 May;29(3):237-45. doi: 10.1055/s-0031-1275517. Epub 2011 Jun 27. Semin Reprod Med. 2011. PMID: 21710399 Free PMC article. Review.
-
Metabolic Implications of Circadian-HIF Crosstalk.Trends Endocrinol Metab. 2020 Jun;31(6):459-468. doi: 10.1016/j.tem.2020.02.008. Epub 2020 Mar 18. Trends Endocrinol Metab. 2020. PMID: 32396846 Review.
Cited by
-
Nutritional Approaches Targeting Gut Microbiota in Oxidative-Stress-Associated Metabolic Syndrome: Focus on Early Life Programming.Nutrients. 2024 Feb 28;16(5):683. doi: 10.3390/nu16050683. Nutrients. 2024. PMID: 38474810 Free PMC article. Review.
-
Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease.Int J Mol Sci. 2020 Mar 23;21(6):2232. doi: 10.3390/ijms21062232. Int J Mol Sci. 2020. PMID: 32210175 Free PMC article. Review.
-
Amino acid metabolism reprogramming: shedding new light on T cell anti-tumor immunity.J Exp Clin Cancer Res. 2023 Nov 3;42(1):291. doi: 10.1186/s13046-023-02845-4. J Exp Clin Cancer Res. 2023. PMID: 37924140 Free PMC article. Review.
-
Melatonin Use during Pregnancy and Lactation Complicated by Oxidative Stress: Focus on Offspring's Cardiovascular-Kidney-Metabolic Health in Animal Models.Antioxidants (Basel). 2024 Feb 12;13(2):226. doi: 10.3390/antiox13020226. Antioxidants (Basel). 2024. PMID: 38397824 Free PMC article. Review.
-
A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle.J Exp Clin Cancer Res. 2024 Mar 8;43(1):74. doi: 10.1186/s13046-024-02994-0. J Exp Clin Cancer Res. 2024. PMID: 38459595 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

