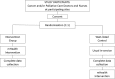
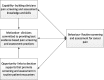
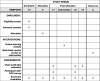
A phase III wait-listed randomised controlled trial of novel targeted inter-professional clinical education intervention to improve cancer patients' reported pain outcomes (The Cancer Pain Assessment (CPAS) Trial): study protocol
- PMID: 30658657
- PMCID: PMC6339283
- DOI: 10.1186/s13063-018-3152-z
A phase III wait-listed randomised controlled trial of novel targeted inter-professional clinical education intervention to improve cancer patients' reported pain outcomes (The Cancer Pain Assessment (CPAS) Trial): study protocol
Abstract
Background: Variations in care models contribute to cancer pain being under-recognised and under-treated in half of all patients with cancer. International and national cancer pain management guidelines are achievable with minimal investment but require practice changes. While much of the cancer pain research over the preceding decades has focused on management interventions, little attention has been given to achieving better adherence to recommended cancer pain guideline screening and assessment practices. This trial aims to reduce unrelieved cancer pain by improving cancer and palliative doctors' and nurses' ('clinicians') pain assessment capabilities through a targeted inter-professional clinical education intervention delivered to participants' mobile devices ('mHealth').
Methods: A wait-listed, randomised control trial design. Cancer and/or palliative care physicians and nurses employed at one of the six participating sites across Australia will be eligible to participate in this trial and, on enrolment, will be allocated to the active or wait-listed arm. Participants allocated to the active arm will be invited to complete the mHealth cancer pain assessment intervention. In this trial, mHealth is defined as medical or public health practice supported by mobile devices (i.e. phones, patient monitoring devices, personal digital assistants and other wireless devices). This mHealth intervention integrates three evidence-based elements, namely: the COM-B theoretical framework; spaced learning pedagogy; and audit and feedback. This intervention will be delivered via the QStream online platform to participants' mobile devices over four weeks. The trial will determine if a tailored mHealth intervention, targeting clinicians' cancer pain assessment capabilities, is effective in reducing self-reported cancer pain scores, as measured by a Numerical Rating Scale (NRS).
Discussion: If this mHealth intervention is found to be effective, in addition to improving cancer pain assessment practices, it will provide a readily transferable evidence-based framework that could readily be applied to other evidence practice gaps and a scalable intervention that could be administered simultaneously to multiple clinicians across diverse geographical locations. Moreover, if found to be cost-effective, it will help transform clinical continuing professional development. In summary, this mHealth intervention will provide health services with an opportunity to offer an evidence-based, pedagogically robust, cost-effective, scalable training alternative.
Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN12618001103257 . Registered on 3 July 2018.
Keywords: Assessment, pain; Cancer pain; Clinical competence; Cost-benefit analysis; Education, professional; Health services research; MeSH terms; Mobile applications; Pain measurement; Palliative care; Patient-reported outcome measures.
Conflict of interest statement
Ethics approval and consent to participate
Research ethics approval was granted by South Eastern Sydney Local Health District Human Research Ethics Committee (SESLHD HREC) on May 30, 2018. HREC reference number: 17/322 (HREC/18/POWH/90).
Proposed protocol amendments will be submitted to SESLHD HREC in the first instance. Following amendment approval, the Co-ordinating Investigator (JP) will communicate protocol amendments in writing to all relevant parties.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32(14):1480–1501. doi: 10.1200/JCO.2013.53.5948. - DOI - PubMed
-
- Etkind SN, Daveson BA, Kwok W, Witt J, Bausewein C, Higginson IJ, et al. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manag. 2014;49(3):611–24. - PubMed
-
- Brink-Huis A, van Achterberg T, Schoonhoven L, Brink-Huis A, van Achterberg T, Schoonhoven L. Pain management: a review of organisation models with integrated processes for the management of pain in adult cancer patients. J Clin Nurs. 2008;17(15):1986–2000. doi: 10.1111/j.1365-2702.2007.02228.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical