Chinese herbal medicine bi min fang for allergic rhinitis: protocol for a double-blind, double-dummy, randomized controlled trial
- PMID: 30658660
- PMCID: PMC6339341
- DOI: 10.1186/s13063-018-3151-0
Chinese herbal medicine bi min fang for allergic rhinitis: protocol for a double-blind, double-dummy, randomized controlled trial
Abstract
Background: People with allergic rhinitis (AR) often seek help from Chinese medicine due to dissatisfaction with conventional treatments. Lung-spleen qi deficiency syndrome (LSQDS) is the most common type of AR, and the Chinese herbal medicine formula bi min fang (BMF) is commonly prescribed for AR patients with LSQDS. However, direct evidence supporting its efficacy and safety is not available, and its potential mechanism of action remains unclear.
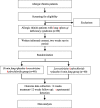
Methods/design: This paper presents a double-blind, double-dummy, randomized controlled trial. After a 2-week run-in period, 80 AR patients with LSQDS will be recruited and randomly allocated to the BMF group or the control group in a 1:1 ratio. The patients in the BMF group will receive BMF and the placebo for levocetirizine hydrochloride orally, while the control group participants will receive levocetirizine hydrochloride and the placebo for BMF orally. All participants will receive 4 weeks of treatment and 12 weeks of follow-up. The primary outcome is a change in the Total Nasal Symptom Score (TNSS). Secondary outcomes include changes in scores for the standard version of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ(S)), and visual analog scale (VAS); changes in serum levels of the cytokines interleukin-4, interferon-γ, transforming growth factor β-1, and interleukin-17; and changes in the gut microbiota composition in the stool. The TNSS, RQLQ(S), and VAS will be recorded at the beginning of, middle of and after the treatment period and at the end of each month in the 3-month follow-up period. Blood and stool samples will be collected at baseline and the end of the treatment. The aforementioned four cytokines will be detected in the serum using enzyme-linked immunosorbent assays, and the stool gut microbiota will be detected using 16S ribosomal ribonucleic acid sequencing. Any side effects of the treatment will be recorded.
Discussion: The results of this trial will provide consolidated evidence of the effect of BMF on AR and the potential mechanism by which BMF acts. This study will be the first to explore the mechanism of action of Chinese herbal medicine on the gut microbiota in AR.
Trial registration: Chinese Clinical Trial Registry, ChiCTR-IPR-17010970 . Registered on 23 March 2017.
Keywords: Allergic rhinitis; Chinese herbal medicine; Cytokines; Gut microbiota; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval for this trial protocol has been obtained from the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (number: B2016-100-01). All participants will be provided with sufficient time to consider whether to enter the trial or not. The investigators and research assistants must obtain the written informed consent from each participant before randomization. The study will be conducted in accordance with the ethical principles of the Declaration of Helsinki (2013 version).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Similar articles
-
Efficacy of Bimin decoction for patients with perennial allergic rhinitis: an open-label non-inferiority randomized controlled trial.Trials. 2019 Dec 30;20(1):802. doi: 10.1186/s13063-019-3763-z. Trials. 2019. PMID: 31888713 Free PMC article. Clinical Trial.
-
Socheongryong-tang for improving nasal symptoms associated with allergic rhinitis: A study protocol for a randomized, open-label, cetirizine controlled, clinical trial.Medicine (Baltimore). 2018 Aug;97(34):e11812. doi: 10.1097/MD.0000000000011812. Medicine (Baltimore). 2018. PMID: 30142767 Free PMC article. Clinical Trial.
-
Efficacy and safety of Shu-gan-qing-re formula for generalized anxiety disorder: study protocol for a multi-center, prospective, double-blind, double-dummy, randomized controlled trial.Trials. 2020 Mar 14;21(1):266. doi: 10.1186/s13063-020-4186-6. Trials. 2020. PMID: 32171323 Free PMC article.
-
Efficacy and safety of acupoint application for allergic rhinitis: a systematic review and Meta-analysis of randomized controlled trials.J Tradit Chin Med. 2022 Dec;42(6):858-868. doi: 10.19852/j.cnki.jtcm.2022.06.003. J Tradit Chin Med. 2022. PMID: 36378042 Free PMC article.
-
Recent Patents of Complementary and Alternative Medicine for Allergic Rhinitis.Recent Pat Inflamm Allergy Drug Discov. 2015;9(2):107-19. doi: 10.2174/1872213x10666151119144718. Recent Pat Inflamm Allergy Drug Discov. 2015. PMID: 26581315 Review.
Cited by
-
The efficacy and safety of Yupingfeng Powder with variation in the treatment of allergic rhinitis: Study protocol for a randomized, double-blind, placebo-controlled trial.Front Pharmacol. 2022 Dec 16;13:1058176. doi: 10.3389/fphar.2022.1058176. eCollection 2022. Front Pharmacol. 2022. PMID: 36588672 Free PMC article.
References
-
- Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association Chinese guidelines for diagnosis and treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51(1):6–24. - PubMed
-
- Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(Suppl 2):12–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials