Screening for differentially expressed miRNAs in Aedes albopictus (Diptera: Culicidae) exposed to DENV-2 and their effect on replication of DENV-2 in C6/36 cells
- PMID: 30658692
- PMCID: PMC6339288
- DOI: 10.1186/s13071-018-3261-2
Screening for differentially expressed miRNAs in Aedes albopictus (Diptera: Culicidae) exposed to DENV-2 and their effect on replication of DENV-2 in C6/36 cells
Abstract
Background: The mosquito Aedes albopictus is an important vector for dengue virus (DENV) transmission. The midgut is the first barrier to mosquito infection by DENV, and this barrier is a critical factor affecting the vector competence of the mosquito. However, the molecular mechanism of the interaction between midgut and virus is unknown.
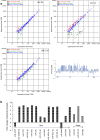
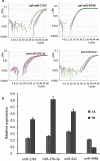
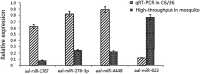
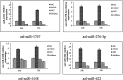
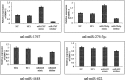
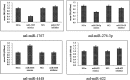
Results: Six small libraries of Ae. albopictus midgut RNAs were constructed, three of which from mosquitoes that were infected with DENV-2 after feeding on infected blood, and another three that remained uninfected with DENV-2 after feeding on same batch of infected blood. A total of 46 differentially expressed miRNAs were identified of which 17 significant differentially expressed miRNAs were selected. Compared to microRNA expression profiles of mosquitoes that were uninfected with DENV-2, 15 microRNAs were upregulated and two were downregulated in mosquitoes that were infected with DENV-2. Among these differentially expressed microRNAs, miR-1767, miR-276-3p, miR-4448 and miR-622 were verified by stem-loop qRT-PCR in samples from seven-day-infected and uninfected midguts and chosen for an in vitro transient transfection assay. miR-1767 and miR-276-3p enhanced dengue virus replication in C6/36 cells, and miR-4448 reduced dengue virus replication.
Conclusions: To our knowledge, this study is the first to reveal differences in expression levels between mosquitoes infected and uninfected with DENV-2 after feeding on an infected blood meal. It provides useful information on microRNAs expressed in the midgut of Aedes albopictus after exposure to the virus.
Keywords: Aedes albopictus; Dengue virus (DENV); Midgut; microRNA (miRNA).
Conflict of interest statement
Ethics approval
All of the experimental protocols involving animals were approved by the Laboratory Animal Center of the State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology Institutional Animal Care and Use Committee (IACUC, permit number BIME 2011-2009). The study of animals was performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Identification of microRNAs expressed in the midgut of Aedes albopictus during dengue infection.Parasit Vectors. 2017 Feb 3;10(1):63. doi: 10.1186/s13071-017-1966-2. Parasit Vectors. 2017. PMID: 28159012 Free PMC article.
-
piRNA Profiling of Dengue Virus Type 2-Infected Asian Tiger Mosquito and Midgut Tissues.Viruses. 2018 Apr 22;10(4):213. doi: 10.3390/v10040213. Viruses. 2018. PMID: 29690553 Free PMC article.
-
miR-281, an abundant midgut-specific miRNA of the vector mosquito Aedes albopictus enhances dengue virus replication.Parasit Vectors. 2014 Oct 22;7:488. doi: 10.1186/s13071-014-0488-4. Parasit Vectors. 2014. PMID: 25331963 Free PMC article.
-
Aedes vittatus (Bigot) mosquito: An emerging threat to public health.J Vector Borne Dis. 2017 Oct-Dec;54(4):295-300. doi: 10.4103/0972-9062.225833. J Vector Borne Dis. 2017. PMID: 29460858 Review.
-
Molecular pathogenesis of dengue virus infection in Aedes mosquitoes.J Insect Physiol. 2022 Apr;138:104367. doi: 10.1016/j.jinsphys.2022.104367. Epub 2022 Feb 4. J Insect Physiol. 2022. PMID: 35131236 Review.
Cited by
-
Aedes albopictus salivary adenosine deaminase is an immunomodulatory factor facilitating dengue virus replication.Sci Rep. 2023 Oct 4;13(1):16660. doi: 10.1038/s41598-023-43751-1. Sci Rep. 2023. PMID: 37794048 Free PMC article.
-
Tropical Medicine in China: Bibliometric Analysis Based on Web of Science (2010-2019).J Trop Med. 2021 Aug 10;2021:4267230. doi: 10.1155/2021/4267230. eCollection 2021. J Trop Med. 2021. PMID: 34422063 Free PMC article.
-
Filtering the Junk: Assigning Function to the Mosquito Non-Coding Genome.Insects. 2021 Feb 22;12(2):186. doi: 10.3390/insects12020186. Insects. 2021. PMID: 33671692 Free PMC article. Review.
-
Evaluation of Exosomal miRNA in Blood as a Potential Diagnostic Biomarker for Human Non-Small Cell Lung Cancer.Med Sci Monit. 2020 May 23;26:e924721. doi: 10.12659/MSM.924721. Med Sci Monit. 2020. PMID: 32444593 Free PMC article.
-
Response of the mosquito immune system and symbiotic bacteria to pathogen infection.Parasit Vectors. 2024 Feb 17;17(1):69. doi: 10.1186/s13071-024-06161-4. Parasit Vectors. 2024. PMID: 38368353 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

