Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial
- PMID: 30658932
- PMCID: PMC6701190
- DOI: 10.1016/S1470-2045(18)30778-2
Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial
Erratum in
-
Correction to Lancet Oncol 2019; 20: 297-310.Lancet Oncol. 2019 Jun;20(6):e293. doi: 10.1016/S1470-2045(19)30348-1. Lancet Oncol. 2019. PMID: 31162102 No abstract available.
Abstract
Background: In the ongoing phase 3, CheckMate 214 trial, nivolumab plus ipilimumab improved overall survival compared with sunitinib in patients with intermediate or poor risk, previously untreated, advanced renal cell carcinoma. We aimed to assess whether health-related quality of life (HRQoL) could be used to further describe the benefit-risk profile of nivolumab plus ipilimumab versus sunitinib.
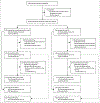
Methods: In the phase 3, randomised, controlled, CheckMate 214 trial, patients aged 18 years and older with previously untreated, advanced or metastatic renal cell carcinoma with a clear-cell component were recruited from 175 hospitals and cancer centres in 28 countries. Patients were categorised by risk status into favourable, intermediate, and poor risk subgroups and randomly assigned (1:1) to open-label nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab 3 mg/kg every 2 weeks, or sunitinib 50 mg/day for 4 weeks of each 6-week cycle. Randomisation was done with a block size of four and stratified by risk status and geographical region. Patient-reported outcomes (PROs) were assessed using the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 (FKSI-19), Functional Assessment of Cancer Therapy-General (FACT-G), and EuroQol five dimensional three level (EQ-5D-3L) instruments. The coprimary endpoints of the trial, reported previously, were overall survival, progression-free survival, and the proportion of patients who had an objective response in those categorised as at intermediate or poor risk. PROs in all randomised participants were assessed as an exploratory endpoint; here we report this exploratory endpoint. This study is registered with ClinicalTrials.gov, number NCT02231749, and is ongoing but is now closed to recruitment.
Findings: Between Oct 16, 2014, and Feb 23, 2016, of 1390 patients screened, 1096 (79%) were randomly assigned to treatment, of whom 847 (77%) were at intermediate or poor risk and randomly assigned to nivolumab plus ipilimumab (n=425) or sunitinib (n=422). Median follow-up was 25·2 months (IQR 23·0-27·4). PROs were more favourable with nivolumab plus ipilimumab than sunitinib throughout the first 103 weeks after baseline, with mean change from baseline at week 103 for FKSI-19 total score being 4·00 (95% CI 1·91 to 6·09) for nivolumab plus ipilimumab versus -3·14 (-6·03 to -0·25) for sunitinib (p<0·0001), and for FACT-G total score being 4·77 (1·73 to 7·82) for nivolumab plus ipilimumab versus -4·32 (-8·54 to -0·11) for sunitinib (p=0·0005). Significant differences were also seen for four of five FKSI-19 domains (disease-related symptoms, physical disease-related symptoms, treatment side-effects, and functional wellbeing) and FACT-G physical and functional wellbeing domains. However, there was no significant difference between the treatment groups at week 103 in EQ-5D-3L visual analogue rating scale (VAS) scores, with mean change from baseline to week 103 of 10·07 (95% CI 4·35 to 15·80) for nivolumab plus ipilimumab and 6·40 (-1·36 to 14·16) for sunitinib (p=0·45). Compared with sunitinib, nivolumab plus ipilimumab reduced risk of deterioration in FKSI-19 total score (hazard ratio [HR] 0·54; 95% CI 0·46-0·63), FACT-G total score (0·63, 0·52-0·75), and EQ-5D-3L VAS score (HR 0·75, 95% CI 0·63-0·89) and UK utility scores (0·67, 0·57-0·80).
Interpretation: Nivolumab plus ipilimumab leads to fewer symptoms and better HRQoL than sunitinib in patients at intermediate or poor risk with advanced renal cell carcinoma. These results suggest that the superior efficacy of nivolumab plus ipilimumab over sunitinib comes with the additional benefit of improved HRQoL.
Funding: Bristol-Myers Squibb and ONO Pharmaceutical.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
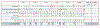


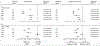
Comment in
-
CheckMate 214 patient-reported outcomes: listening to our patients.Lancet Oncol. 2019 Feb;20(2):179-180. doi: 10.1016/S1470-2045(18)30790-3. Epub 2019 Jan 15. Lancet Oncol. 2019. PMID: 30658937 No abstract available.
References
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017; 376: 354–66. - PubMed
-
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–99. - PubMed
-
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

