To Fix or Not To Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere
- PMID: 30658971
- PMCID: PMC6414387
- DOI: 10.1128/AEM.02546-18
To Fix or Not To Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere
Erratum in
-
Erratum for Smercina et al., "To Fix or Not To Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere".Appl Environ Microbiol. 2019 Oct 30;85(22):e02103-19. doi: 10.1128/AEM.02103-19. Print 2019 Nov 15. Appl Environ Microbiol. 2019. PMID: 31666239 Free PMC article. No abstract available.
Abstract
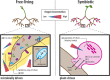
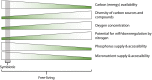
Free-living nitrogen fixation (FLNF) in the rhizosphere, or N fixation by heterotrophic bacteria living on/near root surfaces, is ubiquitous and a significant source of N in some terrestrial systems. FLNF is also of interest in crop production as an alternative to chemical fertilizer, potentially reducing production costs and ameliorating negative environmental impacts of fertilizer N additions. Despite this interest, a mechanistic understanding of controls (e.g., carbon, oxygen, nitrogen, and nutrient availability) on FLNF in the rhizosphere is lacking but necessary. FLNF is distinct from and occurs under more diverse and dynamic conditions than symbiotic N fixation; therefore, predicting FLNF rates and understanding controls on FLNF has proven difficult. This has led to large gaps in our understanding of FLNF, and studies aimed at identifying controls on FLNF are needed. Here, we provide a mechanistic overview of FLNF, including how various controls may influence FLNF in the rhizosphere in comparison with symbiotic N fixation occurring in plant nodules where environmental conditions are moderated by the plant. We apply this knowledge to a real-world example, the bioenergy crop switchgrass (Panicum virgatum), to provide context of how FLNF may function in a managed system. We also highlight future challenges to assessing FLNF and understanding how FLNF functions in the environment and significantly contributes to plant N availability and productivity.
Keywords: diazotrophs; environmental controls; free-living nitrogen fixation; rhizosphere; rhizosphere-inhabiting microbes.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI. 2002. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57:1–45. doi: 10.1023/A:1015798428743. - DOI
-
- Reed SC, Cleveland CC, Townsend AR. 2011. Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512. doi: 10.1146/annurev-ecolsys-102710-145034. - DOI
-
- Mus F, Crook MB, Garcia K, Garcia Costas A, Geddes BA, Kouri ED, Paramasivan P, Ryu M-H, Oldroyd GED, Poole PS, Udvardi MK, Voigt CA, Ané J-M, Peters JW. 2016. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl Environ Microbiol 82:3698–3710. doi: 10.1128/AEM.01055-16. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

