Improved n-Butanol Production from Clostridium cellulovorans by Integrated Metabolic and Evolutionary Engineering
- PMID: 30658972
- PMCID: PMC6585503
- DOI: 10.1128/AEM.02560-18
Improved n-Butanol Production from Clostridium cellulovorans by Integrated Metabolic and Evolutionary Engineering
Abstract
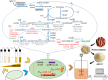
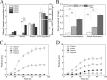
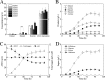
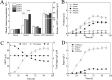
Clostridium cellulovorans DSM 743B offers potential as a chassis strain for biomass refining by consolidated bioprocessing (CBP). However, its n-butanol production from lignocellulosic biomass has yet to be demonstrated. This study demonstrates the construction of a coenzyme A (CoA)-dependent acetone-butanol-ethanol (ABE) pathway in C. cellulovorans by introducing adhE1 and ctfA-ctfB-adc genes from Clostridium acetobutylicum ATCC 824, which enabled it to produce n-butanol using the abundant and low-cost agricultural waste of alkali-extracted, deshelled corn cobs (AECC) as the sole carbon source. Then, a novel adaptive laboratory evolution (ALE) approach was adapted to strengthen the n-butanol tolerance of C. cellulovorans to fully utilize its n-butanol output potential. To further improve n-butanol production, both metabolic engineering and evolutionary engineering were combined, using the evolved strain as a host for metabolic engineering. The n-butanol production from AECC of the engineered C. cellulovorans was increased 138-fold, from less than 0.025 g/liter to 3.47 g/liter. This method represents a milestone toward n-butanol production by CBP, using a single recombinant clostridium strain. The engineered strain offers a promising CBP-enabling microbial chassis for n-butanol fermentation from lignocellulose.IMPORTANCE Due to a lack of genetic tools, Clostridium cellulovorans DSM 743B has not been comprehensively explored as a putative strain platform for n-butanol production by consolidated bioprocessing (CBP). Based on the previous study of genetic tools, strain engineering of C. cellulovorans for the development of a CBP-enabling microbial chassis was demonstrated in this study. Metabolic engineering and evolutionary engineering were integrated to improve the n-butanol production of C. cellulovorans from the low-cost renewable agricultural waste of alkali-extracted, deshelled corn cobs (AECC). The n-butanol production from AECC was increased 138-fold, from less than 0.025 g/liter to 3.47 g/liter, which represents the highest titer of n-butanol produced using a single recombinant clostridium strain by CBP reported to date. This engineered strain serves as a promising chassis for n-butanol production from lignocellulose by CBP.
Keywords: Clostridium; adaptive laboratory evolution; consolidated bioprocessing; metabolic engineering; n-butanol.
Copyright © 2019 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

