Molecular Hydrogen, a Neglected Key Driver of Soil Biogeochemical Processes
- PMID: 30658976
- PMCID: PMC6414374
- DOI: 10.1128/AEM.02418-18
Molecular Hydrogen, a Neglected Key Driver of Soil Biogeochemical Processes
Abstract
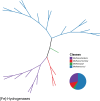
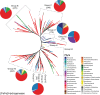
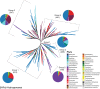
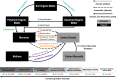
The atmosphere of the early Earth is hypothesized to have been rich in reducing gases such as hydrogen (H2). H2 has been proposed as the first electron donor leading to ATP synthesis due to its ubiquity throughout the biosphere as well as its ability to easily diffuse through microbial cells and its low activation energy requirement. Even today, hydrogenase enzymes enabling the production and oxidation of H2 are found in thousands of genomes spanning the three domains of life across aquatic, terrestrial, and even host-associated ecosystems. Even though H2 has already been proposed as a universal growth and maintenance energy source, its potential contribution as a driver of biogeochemical cycles has received little attention. Here, we bridge this knowledge gap by providing an overview of the classification, distribution, and physiological role of hydrogenases. Distribution of these enzymes in various microbial functional groups and recent experimental evidence are finally integrated to support the hypothesis that H2-oxidizing microbes are keystone species driving C cycling along O2 concentration gradients found in H2-rich soil ecosystems. In conclusion, we suggest focusing on the metabolic flexibility of H2-oxidizing microbes by combining community-level and individual-level approaches aiming to decipher the impact of H2 on C cycling and the C-cycling potential of H2-oxidizing microbes, via both culture-dependent and culture-independent methods, to give us more insight into the role of H2 as a driver of biogeochemical processes.
Keywords: H2 oxidation; anaerobic processes; biogeochemical processes; carbon cycle; environmental microbiology; hydrogen; soil.
Copyright © 2019 American Society for Microbiology.
Figures
References
-
- Oparin AI. 1938. The origin of life. J Chem Educ 15:399. doi: 10.1021/ed015p399.1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

