A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403
- PMID: 30658992
- PMCID: PMC6450431
- DOI: 10.1182/blood-2018-10-881961
A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403
Erratum in
-
Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548-1559.Blood. 2019 Sep 26;134(13):1111. doi: 10.1182/blood.2019002613. Blood. 2019. PMID: 31558558 Free PMC article. No abstract available.
Abstract
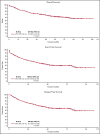
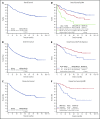
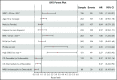
Retrospective studies have suggested that older adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL) have better survival rates when treated using a pediatric ALL regimen administered by pediatric treatment teams. To address the feasibility and efficacy of using a pediatric treatment regimen for AYA patients with newly diagnosed ALL administered by adult treatment teams, we performed a prospective study, CALGB 10403, with doses and schedule identical to those in the Children's Oncology Group study AALL0232. From 2007 to 2012, 318 patients were enrolled; 295 were eligible and evaluable for response. Median age was 24 years (range, 17-39 years). Use of the pediatric regimen was safe; overall treatment-related mortality was 3%, and there were only 2 postremission deaths. Median event-free survival (EFS) was 78.1 months (95% confidence interval [CI], 41.8 to not reached), more than double the historical control of 30 months (95% CI, 22-38 months); 3-year EFS was 59% (95% CI, 54%-65%). Median overall survival (OS) was not reached. Estimated 3-year OS was 73% (95% CI, 68%-78%). Pretreatment risk factors associated with worse treatment outcomes included obesity and presence of the Philadelphia-like gene expression signature. Use of a pediatric regimen for AYAs with ALL up to age 40 years was feasible and effective, resulting in improved survival rates compared with historical controls. CALGB 10403 can be considered a new treatment standard upon which to build for improving survival for AYAs with ALL. This trial was registered at www.clinicaltrials.gov as #NCT00558519.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: W.S. served on advisory boards for Pfizer, Amgen, and Jazz Pharmaceuticals; S.M.L. received clinical trial support from Onconova, Celgene, Cyclacel, Hoffman-La Roche, Onconova, Incyte, Biosight, and Kura and served on an advisory board for Pfizer; A.S.A. received honoraria from Pfizer, Sigma Tau, and Jazz Pharmaceuticals; C.G.M. received honoraria from Amgen and Pfizer; E. Parker owns stock in Teva Pharmaceuticals and Johnson & Johnson; M.S.T. received research funding from AbbVie, AROG, Cellerant, Orsenix, ADC Therapeutics, and Biosight and served on advisory boards for Daiicho-Sankyo, Orsenix, KAHR, Rigel, Abbvie, and Nohla. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Trends in survival of young adult patients with acute lymphoblastic leukemia in Sweden and the United States.Blood. 2019 Jul 25;134(4):407-410. doi: 10.1182/blood.2019001168. Epub 2019 Apr 25. Blood. 2019. PMID: 31023701 No abstract available.
References
-
- Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B study 8811. Blood. 1995;85(8):2025-2037. - PubMed
-
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. - PubMed
-
- Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547-561. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U10 CA180821/CA/NCI NIH HHS/United States
- R50 CA211542/CA/NCI NIH HHS/United States
- U10 CA180836/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA233373/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- UG1 CA189869/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

