Large-scale discovery of mouse transgenic integration sites reveals frequent structural variation and insertional mutagenesis
- PMID: 30659012
- PMCID: PMC6396414
- DOI: 10.1101/gr.233866.117
Large-scale discovery of mouse transgenic integration sites reveals frequent structural variation and insertional mutagenesis
Abstract
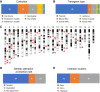
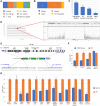
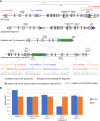
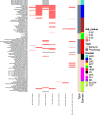
Transgenesis has been a mainstay of mouse genetics for over 30 yr, providing numerous models of human disease and critical genetic tools in widespread use today. Generated through the random integration of DNA fragments into the host genome, transgenesis can lead to insertional mutagenesis if a coding gene or an essential element is disrupted, and there is evidence that larger scale structural variation can accompany the integration. The insertion sites of only a tiny fraction of the thousands of transgenic lines in existence have been discovered and reported, due in part to limitations in the discovery tools. Targeted locus amplification (TLA) provides a robust and efficient means to identify both the insertion site and content of transgenes through deep sequencing of genomic loci linked to specific known transgene cassettes. Here, we report the first large-scale analysis of transgene insertion sites from 40 highly used transgenic mouse lines. We show that the transgenes disrupt the coding sequence of endogenous genes in half of the lines, frequently involving large deletions and/or structural variations at the insertion site. Furthermore, we identify a number of unexpected sequences in some of the transgenes, including undocumented cassettes and contaminating DNA fragments. We demonstrate that these transgene insertions can have phenotypic consequences, which could confound certain experiments, emphasizing the need for careful attention to control strategies. Together, these data show that transgenic alleles display a high rate of potentially confounding genetic events and highlight the need for careful characterization of each line to assure interpretable and reproducible experiments.
© 2019 Goodwin et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S, Wadugu B, Arab S, Kuhn B. 2013. Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis Model Mech 6: 1459–1469. 10.1242/dmm.010447 - DOI - PMC - PubMed
-
- Cain-Hom C, Splinter E, van Min M, Simonis M, van de Heijning M, Martinez M, Asghari V, Cox JC, Warming S. 2017. Efficient mapping of transgene integration sites and local structural changes in Cre transgenic mice using targeted locus amplification. Nucleic Acids Res 45: e62 10.1093/nar/gkw1329 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
