MNK1/NODAL Signaling Promotes Invasive Progression of Breast Ductal Carcinoma In Situ
- PMID: 30659022
- PMCID: PMC6513674
- DOI: 10.1158/0008-5472.CAN-18-1602
MNK1/NODAL Signaling Promotes Invasive Progression of Breast Ductal Carcinoma In Situ
Erratum in
-
Correction: MNK1/NODAL Signaling Promotes Invasive Progression of Breast Ductal Carcinoma In Situ.Cancer Res. 2024 Apr 15;84(8):1373. doi: 10.1158/0008-5472.CAN-24-0461. Cancer Res. 2024. PMID: 38616659 No abstract available.
Abstract
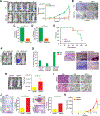
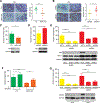
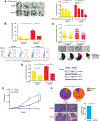
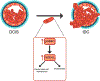
The mechanisms by which breast cancers progress from relatively indolent ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC) are not well understood. However, this process is critical to the acquisition of metastatic potential. MAPK-interacting serine/threonine-protein kinase 1 (MNK1) signaling can promote cell invasion. NODAL, a morphogen essential for embryogenic patterning, is often reexpressed in breast cancer. Here we describe a MNK1/NODAL signaling axis that promotes DCIS progression to IDC. We generated MNK1 knockout (KO) or constitutively active MNK1 (caMNK1)-expressing human MCF-10A-derived DCIS cell lines, which were orthotopically injected into the mammary glands of mice. Loss of MNK1 repressed NODAL expression, inhibited DCIS to IDC conversion, and decreased tumor relapse and metastasis. Conversely, caMNK1 induced NODAL expression and promoted IDC. The MNK1/NODAL axis promoted cancer stem cell properties and invasion in vitro. The MNK1/2 inhibitor SEL201 blocked DCIS progression to invasive disease in vivo. In clinical samples, IDC and DCIS with microinvasion expressed higher levels of phospho-MNK1 and NODAL versus low-grade (invasion-free) DCIS. Cumulatively, our data support further development of MNK1 inhibitors as therapeutics for preventing invasive disease. SIGNIFICANCE: These findings provide new mechanistic insight into progression of ductal carcinoma and support clinical application of MNK1 inhibitors to delay progression of indolent ductal carcinoma in situ to invasive ductal carcinoma.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures


References
-
- Martinez-Perez C, Turnbull AK, Ekatah GE, Arthur LM, Sims AH, Thomas JS, et al. Current treatment trends and the need for better predictive tools in the management of ductal carcinoma in situ of the breast. Cancer Treat Rev 2017;55:163–72. - PubMed
-
- Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, et al. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem 1998; 273:9373–7. - PubMed
-
- Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov 2015; 14:261–78. - PubMed
-
- Adesso L, Calabretta S, Barbagallo F, Capurso G, Pilozzi E, Geremia R, et al. Gemcitabine triggers a pro-survival response in pancreatic cancer cells through activation of the MNK2/eIF4E pathway. Oncogene 2013;32:2848–57. - PubMed
-
- Chrestensen CA, Shuman JK, Eschenroeder A, Worthington M, Gram H, Sturgill TW. MNK1 and MNK2 regulation in HER2-overexpressing breast cancer lines. J Biol Chem 2007;282:4243–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

