Folding-function relationship of the most common cystic fibrosis-causing CFTR conductance mutants
- PMID: 30659068
- PMCID: PMC6339265
- DOI: 10.26508/lsa.201800172
Folding-function relationship of the most common cystic fibrosis-causing CFTR conductance mutants
Abstract
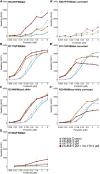
Cystic fibrosis is caused by mutations in the CFTR gene, which are subdivided into six classes. Mutants of classes III and IV reach the cell surface but have limited function. Most class-III and class-IV mutants respond well to the recently approved potentiator VX-770, which opens the channel. We here revisited function and folding of some class-IV mutants and discovered that R347P is the only one that leads to major defects in folding. By this criterion and by its functional response to corrector drug VX-809, R347P qualifies also as a class-II mutation. Other class-IV mutants folded like wild-type CFTR and responded similarly to VX-809, demonstrating how function and folding are connected. Studies on both types of defects complement each other in understanding how compounds improve mutant CFTR function. This provides an attractive unbiased approach for characterizing mode of action of novel therapeutic compounds and helps address which drugs are efficacious for each cystic fibrosis disease variant.
© 2019 van Willigen et al.
Conflict of interest statement
CK van der Ent received funding from Vertex Pharmaceuticals.
Figures




References
-
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. (1997) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 15: 236–246. 10.1038/ng0397-236 - DOI - PubMed
-
- Chen PC, Olson EM, Zhou Q, Kryukova Y, Sampson HM, Thomas DY, Shyng SL (2013) Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J Biol Chem 288: 20942–20954. 10.1074/jbc.m113.470948 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
