Hepatitis B virus X protein-induced SH2 domain-containing 5 (SH2D5) expression promotes hepatoma cell growth via an SH2D5-transketolase interaction
- PMID: 30659097
- PMCID: PMC6442033
- DOI: 10.1074/jbc.RA118.005739
Hepatitis B virus X protein-induced SH2 domain-containing 5 (SH2D5) expression promotes hepatoma cell growth via an SH2D5-transketolase interaction
Abstract
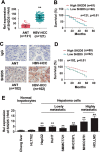
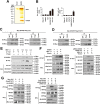
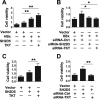
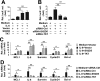
Hepatitis B virus X protein (HBx) critically contributes to the development of hepatocellular carcinoma (HCC). However, the mechanisms by which HBx promotes HCC remain unclear. In the present study, using a combination of gene expression profiling and immunohistochemistry, we found higher levels of SH2 domain-containing 5 (SH2D5) in liver tissue from HBV-associated HCC (HBV-HCC) patients than in adjacent nontumor tissues. Moreover, HBV infection elevated SH2D5 levels, and we observed that HBx plays an important role in SH2D5 induction. We also found that HBx triggers SH2D5 expression through the NF-κB and c-Jun kinase pathways. Employing SH2D5 overexpression or knockdown, we further demonstrate that SH2D5 promotes HCC cell proliferation both in vitro and in vivo While investigating the mechanism of SH2D5-mediated stimulation of HCC cell proliferation, we noted that HBV induces SH2D5 binding to transketolase (TKT), a pentose phosphate pathway enzyme, thereby promoting an interaction between and signal transducer and activator of transcription 3 (STAT3). Furthermore, HBx stimulated STAT3 phosphorylation at Tyr-705 and promoted the activity and downstream signaling pathway of STAT3 via the SH2D5-TKT interaction. Taken together, our results suggest that SH2D5 is an HBV-induced protein capable of binding to TKT, leading to induction of HCC cell proliferation.
Keywords: HBV X protein; HBV-associated HCC; IL-6-STAT3 signaling; SH2D5; cancer biology; cell migration; hepatitis B virus (HBV, Hep B); inflammation; interleukin 6 (IL-6); transketolase.
© 2019 Zheng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Chen S., Dong Z., Yang P., Wang X., Jin G., Yu H., Chen L., Li L., Tang L., Bai S., Yan H., Shen F., Cong W., Wen W., and Wang H. (2017) Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis. Cancer Lett. 394, 22–32 10.1016/j.canlet.2017.02.011 - DOI - PubMed
-
- Yang P., Li Q. J., Feng Y., Zhang Y., Markowitz G. J., Ning S., Deng Y., Zhao J., Jiang S., Yuan Y., Wang H. Y., Cheng S. Q., Xie D., and Wang X. F. (2012) TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 22, 291–303 10.1016/j.ccr.2012.07.023 - DOI - PMC - PubMed
-
- Liu D., Cui L., Wang Y., Yang G., He J., Hao R., Fan C., Qu M., Liu Z., Wang M., Chen L., Li H., and Guo D. (2016) Hepatitis B e antigen and its precursors promote the progress of hepatocellular carcinoma by interacting with NUMB and decreasing p53 activity. Hepatology 64, 390–404 10.1002/hep.28594 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

