An amphipathic helix enables septins to sense micrometer-scale membrane curvature
- PMID: 30659102
- PMCID: PMC6446858
- DOI: 10.1083/jcb.201807211
An amphipathic helix enables septins to sense micrometer-scale membrane curvature
Abstract
Cell shape is well described by membrane curvature. Septins are filament-forming, GTP-binding proteins that assemble on positive, micrometer-scale curvatures. Here, we examine the molecular basis of curvature sensing by septins. We show that differences in affinity and the number of binding sites drive curvature-specific adsorption of septins. Moreover, we find septin assembly onto curved membranes is cooperative and show that geometry influences higher-order arrangement of septin filaments. Although septins must form polymers to stay associated with membranes, septin filaments do not have to span micrometers in length to sense curvature, as we find that single-septin complexes have curvature-dependent association rates. We trace this ability to an amphipathic helix (AH) located on the C-terminus of Cdc12. The AH domain is necessary and sufficient for curvature sensing both in vitro and in vivo. These data show that curvature sensing by septins operates at much smaller length scales than the micrometer curvatures being detected.
© 2019 Cannon et al.
Figures
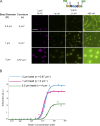
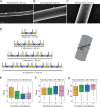

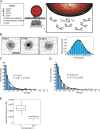

Comment in
-
The long and short of membrane curvature sensing by septins.J Cell Biol. 2019 Apr 1;218(4):1083-1085. doi: 10.1083/jcb.201903045. Epub 2019 Mar 19. J Cell Biol. 2019. PMID: 30890557 Free PMC article.
Similar articles
-
Interplay of septin amphipathic helices in sensing membrane-curvature and filament bundling.Mol Biol Cell. 2021 Oct 1;32(20):br5. doi: 10.1091/mbc.E20-05-0303. Epub 2021 Jul 28. Mol Biol Cell. 2021. PMID: 34319771 Free PMC article.
-
Curvature sensing as an emergent property of multiscale assembly of septins.Proc Natl Acad Sci U S A. 2023 Feb 7;120(6):e2208253120. doi: 10.1073/pnas.2208253120. Epub 2023 Jan 30. Proc Natl Acad Sci U S A. 2023. PMID: 36716363 Free PMC article.
-
Septin phosphorylation and coiled-coil domains function in cell and septin ring morphology in the filamentous fungus Ashbya gossypii.Eukaryot Cell. 2013 Feb;12(2):182-93. doi: 10.1128/EC.00251-12. Epub 2012 Nov 30. Eukaryot Cell. 2013. PMID: 23204191 Free PMC article.
-
Drivers of Morphogenesis: Curvature Sensor Self-Assembly at the Membrane.Cold Spring Harb Perspect Biol. 2024 Dec 2;16(12):a041528. doi: 10.1101/cshperspect.a041528. Cold Spring Harb Perspect Biol. 2024. PMID: 38697653 Review.
-
[Functional Characterization of Septin Complexes].Mol Biol (Mosk). 2018 Mar-Apr;52(2):155-171. doi: 10.7868/S0026898418020015. Mol Biol (Mosk). 2018. PMID: 29695686 Free PMC article. Review.
Cited by
-
Masters of asymmetry - lessons and perspectives from 50 years of septins.Mol Biol Cell. 2020 Oct 1;31(21):2289-2297. doi: 10.1091/mbc.E19-11-0648. Mol Biol Cell. 2020. PMID: 32991244 Free PMC article. Review.
-
The Trypanosoma brucei subpellicular microtubule array is organized into functionally discrete subdomains defined by microtubule associated proteins.PLoS Pathog. 2021 May 19;17(5):e1009588. doi: 10.1371/journal.ppat.1009588. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34010336 Free PMC article.
-
Discovery of broad-spectrum fungicides that block septin-dependent infection processes of pathogenic fungi.Nat Microbiol. 2020 Dec;5(12):1565-1575. doi: 10.1038/s41564-020-00790-y. Epub 2020 Sep 21. Nat Microbiol. 2020. PMID: 32958858
-
Caenorhabditis elegans septins contribute to the development and structure of the oogenic germline.Cytoskeleton (Hoboken). 2023 Jul-Aug;80(7-8):215-227. doi: 10.1002/cm.21763. Epub 2023 Jun 2. Cytoskeleton (Hoboken). 2023. PMID: 37265173 Free PMC article.
-
Spatial regulation of microtubule-dependent transport by septin GTPases.Trends Cell Biol. 2021 Dec;31(12):979-993. doi: 10.1016/j.tcb.2021.06.004. Epub 2021 Jul 9. Trends Cell Biol. 2021. PMID: 34253430 Free PMC article. Review.
References
-
- Akil A., Peng J., Omrane M., Gondeau C., Desterke C., Marin M., Tronchère H., Taveneau C., Sar S., Briolotti P., et al. . 2016. Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat. Commun. 7:12203 10.1038/ncomms12203 - DOI - PMC - PubMed
-
- Bertin A., McMurray M.A., Grob P., Park S.-S., Garcia G. III, Patanwala I., Ng H.-L., Alber T., Thorner J., and Nogales E.. 2008. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. USA. 105:8274–8279. 10.1073/pnas.0803330105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

