Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence
- PMID: 30659149
- PMCID: PMC6397547
- DOI: 10.1073/pnas.1817018116
Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence
Abstract
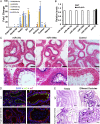
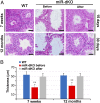
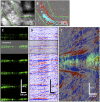
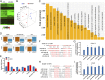
Cilia are cell-surface, microtubule-based organelles that project into extracellular space. Motile cilia are conserved throughout eukaryotes, and their beat induces the flow of fluid, relative to cell surfaces. In mammals, the coordinated beat of motile cilia provides highly specialized functions associated with the movement of luminal contents, as seen with metachronal waves transporting mucus in the respiratory tract. Motile cilia are also present in the male and female reproductive tracts. In the female, wave-like motions of oviductal cilia transport oocytes and embryos toward the uterus. A similar function has been assumed for motile cilia in efferent ductules of the male-i.e., to transport immotile sperm from rete testis into the epididymis. However, we report here that efferent ductal cilia in the male do not display a uniform wave-like beat to transport sperm solely in one direction, but rather exert a centripetal force on luminal fluids through whip-like beating with continual changes in direction, generating turbulence, which maintains immotile spermatozoa in suspension within the lumen. Genetic ablation of two miRNA clusters (miR-34b/c and -449a/b/c) led to failure in multiciliogenesis in murine efferent ductules due to dysregulation of numerous genes, and this mouse model allowed us to demonstrate that loss of efferent duct motile cilia causes sperm aggregation and agglutination, luminal obstruction, and sperm granulomas, which, in turn, induce back-pressure atrophy of the testis and ultimately male infertility.
Keywords: fluid resorption; male infertility; microRNA; multiciliogenesis; reproductive tract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Male reproductive tract cilia beat to a different drummer.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3361-3363. doi: 10.1073/pnas.1900112116. Epub 2019 Jan 28. Proc Natl Acad Sci U S A. 2019. PMID: 30692268 Free PMC article. No abstract available.
References
-
- Wong LB, Miller IF, Yeates DB. Nature of the mammalian ciliary metachronal wave. J Appl Physiol (1985) 1993;75:458–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

