Dissecting Heterosis During the Ear Inflorescence Development Stage in Maize via a Metabolomics-based Analysis
- PMID: 30659214
- PMCID: PMC6338801
- DOI: 10.1038/s41598-018-36446-5
Dissecting Heterosis During the Ear Inflorescence Development Stage in Maize via a Metabolomics-based Analysis
Abstract
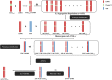
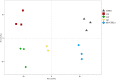
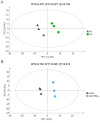
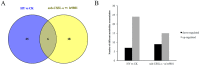
Heterosis can increase the yield of many crops and has been extensively applied in agriculture. In maize, female inflorescence architecture directly determines grain yield. Thus, exploring the relationship between early maize ear inflorescence development and heterosis regarding yield-related traits may be helpful for characterizing the molecular mechanisms underlying heterotic performance. In this study, we fine mapped the overdominant heterotic locus (hlEW2b), associated with ear width, in an approximately 1.98-Mb region based on analyses of chromosome segment substitution lines and the corresponding testcross population. Maize ear inflorescences at the floral meristem stage were collected from two inbred lines, one chromosome segment substitution line that carried hlEW2b (sub-CSSL16), the receptor parent lx9801, and the Zheng58 × sub-CSSL16 and Zheng58 × lx9801 hybrid lines. A total of 256 metabolites were identified, including 31 and 24 metabolites that were differentially accumulated between the two hybrid lines and between the two inbred lines, respectively. Most of these metabolites are involved in complex regulatory mechanisms important for maize ear development. For example, nucleotides are basic metabolites affecting cell composition and carbohydrate synthesis. Additionally, nicotinate and nicotinamide metabolism is important for photosynthesis, plant stress responses, and cell expansion. Moreover, flavonoid and phenolic metabolites regulate auxin transport and cell apoptosis. Meanwhile, phytohormone biosynthesis and distribution influence the cell cycle and cell proliferation. Our results revealed that changes in metabolite contents may affect the heterotic performance related to ear width and yield in maize hybrid lines. This study provides new clues in heterosis at the metabolomics level and implies that differentially accumulated metabolites made distinct contributions to the heterosis at an early stage of ear inflorescences development.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparative transcriptomic analysis of maize ear heterosis during the inflorescence meristem differentiation stage.BMC Plant Biol. 2022 Jul 18;22(1):348. doi: 10.1186/s12870-022-03695-6. BMC Plant Biol. 2022. PMID: 35843937 Free PMC article.
-
Genetic dissection of yield-related traits and mid-parent heterosis for those traits in maize (Zea mays L.).BMC Plant Biol. 2019 Sep 9;19(1):392. doi: 10.1186/s12870-019-2009-2. BMC Plant Biol. 2019. PMID: 31500559 Free PMC article.
-
Heterosis in early maize ear inflorescence development: a genome-wide transcription analysis for two maize inbred lines and their hybrid.Int J Mol Sci. 2014 Aug 11;15(8):13892-915. doi: 10.3390/ijms150813892. Int J Mol Sci. 2014. PMID: 25116687 Free PMC article.
-
Genetic and Molecular Mechanisms of Quantitative Trait Loci Controlling Maize Inflorescence Architecture.Plant Cell Physiol. 2018 Mar 1;59(3):448-457. doi: 10.1093/pcp/pcy022. Plant Cell Physiol. 2018. PMID: 29420811 Review.
-
Genetic Structure and Molecular Mechanisms Underlying the Formation of Tassel, Anther, and Pollen in the Male Inflorescence of Maize (Zea mays L.).Cells. 2022 May 26;11(11):1753. doi: 10.3390/cells11111753. Cells. 2022. PMID: 35681448 Free PMC article. Review.
Cited by
-
Comparative transcriptomic analysis of maize ear heterosis during the inflorescence meristem differentiation stage.BMC Plant Biol. 2022 Jul 18;22(1):348. doi: 10.1186/s12870-022-03695-6. BMC Plant Biol. 2022. PMID: 35843937 Free PMC article.
-
The impact of epistasis in the heterosis and combining ability analyses.Front Plant Sci. 2023 Apr 18;14:1168419. doi: 10.3389/fpls.2023.1168419. eCollection 2023. Front Plant Sci. 2023. PMID: 37143879 Free PMC article.
-
Triploid Hybrid Vigor in Above-Ground Growth and Methane Fermentation Efficiency of Energy Willow.Front Plant Sci. 2022 Feb 23;13:770284. doi: 10.3389/fpls.2022.770284. eCollection 2022. Front Plant Sci. 2022. PMID: 35283877 Free PMC article.
-
A beautiful face is good when we're judged by others, a moral character is better.Soc Cogn Affect Neurosci. 2025 Apr 23;20(1):nsae071. doi: 10.1093/scan/nsae071. Soc Cogn Affect Neurosci. 2025. PMID: 39417256 Free PMC article.
-
Comparative Metabolome and Transcriptome Analysis Reveals the Defense Mechanism of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) against Plasmodiophora brassicae Infection.Int J Mol Sci. 2024 Sep 27;25(19):10440. doi: 10.3390/ijms251910440. Int J Mol Sci. 2024. PMID: 39408769 Free PMC article.
References
-
- Shull GH. The composition of a field of maize. Journal of Heredity. 1908;1:296–301. doi: 10.1093/jhered/os-4.1.296. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

