Neuropsychiatric lupus: new mechanistic insights and future treatment directions
- PMID: 30659245
- PMCID: PMC8023338
- DOI: 10.1038/s41584-018-0156-8
Neuropsychiatric lupus: new mechanistic insights and future treatment directions
Abstract
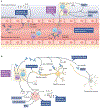
Patients with systemic lupus erythematosus (SLE) frequently show symptoms of central nervous system (CNS) involvement, termed neuropsychiatric SLE (NPSLE). The CNS manifestations of SLE are diverse and have a broad spectrum of severity and prognostic implications. Patients with NPSLE typically present with nonspecific symptoms, such as headache and cognitive impairment, but might also experience devastating features, such as memory loss, seizures and stroke. Some features of NPSLE, in particular those related to coagulopathy, have been characterized and an evidence-based treatment algorithm is available. The cognitive and affective manifestations of NPSLE, however, remain poorly understood. Various immune effectors have been evaluated as contributors to its pathogenesis, including brain-reactive autoantibodies, cytokines and cell-mediated inflammation. Additional brain-intrinsic elements (such as resident microglia, the blood-brain barrier and other neurovascular interfaces) are important facilitators of NPSLE. As yet, however, no unifying model has been found to underlie the pathogenesis of NPSLE, suggesting that this disease has multiple contributors and perhaps several distinct aetiologies. This heterogeneity presents a challenge for clinicians who have traditionally relied on empirical judgement in choosing treatment modalities for patients with NPSLE. Improved understanding of this manifestation of SLE might yield further options for managing this disease.
Figures

References
-
- Unterman A et al. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin. Arthritis Rheum 41, 1–11 (2011). - PubMed
-
- Ainiala H, Loukkola J, Peltola J, Korpela M & Hietaharju A The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 57, 496–500 (2001). - PubMed
-
- Bertsias GK & Boumpas DT Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat. Rev. Rheumatol 6, 358–367 (2010). - PubMed
-
- Borowoy AM et al. Neuropsychiatric lupus: the prevalence and autoantibody associations depend on the definition: results from the 1000 Faces of Lupus cohort. Semin. Arthritis Rheum 42, 179–185 (2012). - PubMed
-
- Kozora E et al. Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus 21, 402–411 (2012). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

