Proper direction of male genitalia is prerequisite for copulation in Drosophila, implying cooperative evolution between genitalia rotation and mating behavior
- PMID: 30659250
- PMCID: PMC6338758
- DOI: 10.1038/s41598-018-36301-7
Proper direction of male genitalia is prerequisite for copulation in Drosophila, implying cooperative evolution between genitalia rotation and mating behavior
Abstract
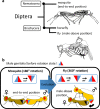
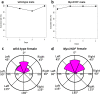
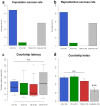
Animal morphology and behavior often appear to evolve cooperatively. However, it is difficult to assess how strictly these two traits depend on each other. The genitalia morphologies and courtship behaviors in insects, which vary widely, may be a good model for addressing this issue. In Diptera, phylogenetic analyses of mating positions suggested that the male-above position evolved from an end-to-end one. However, with this change in mating position, the dorsoventral direction of the male genitalia became upside down with respect to that of the female genitalia. It was proposed that to compensate for this incompatibility, the male genitalia rotated an additional 180° during evolution, implying evolutionary cooperativity between the mating position and genitalia direction. According to this scenario, the proper direction of male genitalia is critical for successful mating. Here, we tested this hypothesis using a Drosophila Myosin31DF (Myo31DF) mutant, in which the rotation of the male genitalia terminates prematurely, resulting in various deviations in genitalia direction. We found that the proper dorsoventral direction of the male genitalia was a prerequisite for successful copulation, but it did not affect the other courtship behaviors. Therefore, our results suggested that the male genitalia rotation and mating position evolved cooperatively in Drosophila.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

