A Novel Mechanism of Renal Microcirculation Regulation: Connecting Tubule-Glomerular Feedback
- PMID: 30659366
- PMCID: PMC6601330
- DOI: 10.1007/s11906-019-0911-5
A Novel Mechanism of Renal Microcirculation Regulation: Connecting Tubule-Glomerular Feedback
Abstract
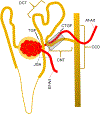
Purpose of review: In this review, we summarized the current knowledge of connecting tubule-glomerular feedback (CTGF), a novel mechanism of renal microcirculation regulation that integrates sodium handling in the connecting tubule (CNT) with kidney hemodynamics.
Recent findings: Connecting tubule-glomerular feedback is a crosstalk communication between the CNT and the afferent arteriole (Af-Art), initiated by sodium chloride through the epithelial sodium channel (ENaC). High sodium in the CNT induces Af-Art vasodilation, increasing glomerular pressure and the glomerular filtration rate and favoring sodium excretion. CTGF antagonized and reset tubuloglomerular feedback and thus increased sodium excretion. CTGF is absent in spontaneous hypertensive rats and is overactivated in Dahl salt-sensitive rats. CTGF is also modulated by angiotensin II and aldosterone. CTGF is a feedback mechanism that integrates sodium handling in the CNT with glomerular hemodynamics. Lack of CTGF could promote hypertension, and CTGF overactivation may favor glomerular damage and proteinuria. More studies are needed to explore the alterations in renal microcirculation and the role of these alterations in the genesis of hypertension and glomerular damage in animals and humans.
Key points: • CTGF is a vasodilator mechanism that regulates afferent arteriole resistance. • CTGF is absent in spontaneous hypertensive rats and overactivated in Dahl salt-sensitive rats. • CTGF in excess may promote glomerular damage and proteinuria, while the absence may participate in sodium retention and hypertension.
Keywords: Connecting tubule-glomerular feedback; ENaC; Hypertension; Proteinuria; Tubuloglomerular feedback.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this manuscript.
Figures

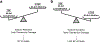

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

