Virus envelope tissue factor promotes infection in mice
- PMID: 30659719
- PMCID: PMC6397068
- DOI: 10.1111/jth.14389
Virus envelope tissue factor promotes infection in mice
Abstract
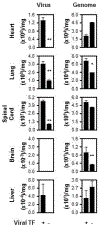
Essentials The coagulation initiator, tissue factor (TF), is on the herpes simplex virus 1 (HSV1) surface. HSV1 surface TF was examined in mice as an antiviral target since it enhances infection in vitro. HSV1 surface TF facilitated infection of all organs evaluated and anticoagulants were antiviral. Protease activated receptor 2 inhibited infection in vivo and its pre-activation was antiviral. SUMMARY: Background Tissue factor (TF) is the essential cell surface initiator of coagulation, and mediates cell signaling through protease-activated receptor (PAR) 2. Having a diverse cellular distribution, TF is involved in many biological pathways and pathologies. Our earlier work identified host cell-derived TF on the envelope covering several viruses, and showed its involvement in enhanced cell infection in vitro. Objective In the current study, we evaluated the in vivo effects of virus surface TF on infection and on the related modulator of infection PAR2. Methods With the use of herpes simplex virus type 1 (HSV1) as a model enveloped virus, purified HSV1 was generated with or without envelope TF through propagation in a TF-inducible cell line. Infection was studied after intravenous inoculation of BALB/c, C57BL/6J or C57BL/6J PAR2 knockout mice with 5 × 105 plaque-forming units of HSV1, mimicking viremia. Three days after inoculation, organs were processed, and virus was quantified with plaque-forming assays and quantitative real-time PCR. Results Infection of brain, lung, heart, spinal cord and liver by HSV1 required viral TF. Demonstrating promise as a therapeutic target, virus-specific anti-TF mAbs or small-molecule inhibitors of coagulation inhibited infection. PAR2 modulates HSV1 in vivo as demonstrated with PAR2 knockout mice and PAR2 agonist peptide. Conclusion TF is a constituent of many permissive host cell types. Therefore, the results presented here may explain why many viruses are correlated with hemostatic abnormalities, and indicate that TF is a novel pan-specific envelope antiviral target.
Keywords: anticoagulant; coagulation; protease-activated receptor; tissue factor; virus infection.
© 2019 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Disclosure of Conflicts of Interests
The authors state that they have no conflict of interest.
Figures




References
-
- Rosen ED, Chan JC, Idusogie E, Clotman F, Vlasuk G, Luther T, Jalbert LR, Albrecht S, Zhong L, Lissens A, Schoonjans L, Moons L, Collen D, Castellino FJ, Carmeliet P. Mice lacking factor VII develop normally but suffer fatal perinatal bleeding. Nature 1997;390:290–294. - PubMed
-
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van V I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D Role of tissue factor in embryonic blood vessel development. Nature 1996;383:73–75. - PubMed
-
- Eisenreich A, Zakrzewicz A, Huber K, Thierbach H, Pepke W, Goldin-Lang P, Schultheiss HP, Pries A, Rauch U. Regulation of pro-angiogenic tissue factor expression in hypoxia-induced human lung cancer cells. Oncol Rep 2013;30:462–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

