Rhinoviruses and Their Receptors
- PMID: 30659817
- PMCID: PMC6533451
- DOI: 10.1016/j.chest.2018.12.012
Rhinoviruses and Their Receptors
Abstract
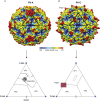
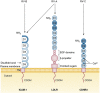
Human rhinoviruses (RVs) are picornaviruses that can cause a variety of upper and lower respiratory tract illnesses, including the common cold, bronchitis, pneumonia, and exacerbations of chronic respiratory diseases such as asthma. There are currently > 160 known types of RVs classified into three species (A, B, and C) that use three different cellular membrane glycoproteins expressed in the respiratory epithelium to enter the host cell. These viral receptors are intercellular adhesion molecule 1 (used by the majority of RV-A and all RV-B types), low-density lipoprotein receptor family members (used by 12 RV-A types), and cadherin-related family member 3 (CDHR3; used by RV-C). RV-A and RV-B interactions with intercellular adhesion molecule 1 and low-density lipoprotein receptor glycoproteins are well defined and their cellular functions have been described, whereas the mechanisms of the RV-C interaction with CDHR3 and its cellular functions are being studied. A single nucleotide polymorphism (rs6967330) in CDHR3 increases cell surface expression of this protein and, as a result, also promotes RV-C infections and illnesses. There are currently no approved vaccines or antiviral therapies available to treat or prevent RV infections, which is a major unmet medical need. Understanding interactions between RV and cellular receptors could lead to new insights into the pathogenesis of respiratory illnesses as well as lead to new approaches to control respiratory illnesses caused by RV infections.
Keywords: antiviral; cadherin-related family member 3; cellular receptor; intercellular adhesion molecule 1; low-density lipoprotein receptor; rhinovirus.
Copyright © 2019 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

