Development and validation of different indirect ELISAs for MERS-CoV serological testing
- PMID: 30659836
- PMCID: PMC7094657
- DOI: 10.1016/j.jim.2019.01.005
Development and validation of different indirect ELISAs for MERS-CoV serological testing
Abstract
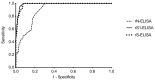
Since 2012, MERS-CoV has caused up to 2220 cases and 790 deaths in 27 countries with Saudi Arabia being the most affected country with ~83.1% of the cases and ~38.8% local death rate. Current serological assays such as microneutralization (MN), plaque reduction neutralization, immunofluorescence, protein microarray or pseudoparticle neutralization assays rely on handling of live MERS-CoV in high containment laboratories or need for expensive and special equipment and reagents and highly trained personnel which represent a technical hurdle for most laboratories in resource-limited MERS-CoV endemic countries. Here, we developed, compared and evaluated three different indirect ELISAs based on MERS-CoV nucleocapsid protein (N), spike (S) ectodomain (amino acids 1-1297) and S1 subunit (amino acids 1-725) and compared them with MN assay. The developed ELISAs were evaluated using large number of confirmed seropositive (79 samples) and seronegative (274 samples) MERS-CoV human serum samples. Both rS1- and rS-ELISAs maintained high sensitivity and specificity (≥90%) across a wider range of OD values compared to rN-ELISA. Moreover, rS1- and rS-based ELISAs showed better agreement and correlation with MN assay in contrast to rN-ELISA. Collectively, our data demonstrate that rS1-ELISA and rS-ELISA are more reliable than rN-ELISA and represent a suitable choice for seroepidemiological testing and surveillance in MERS-CoV endemic regions.
Keywords: ELISA; MERS-CoV; Saudi Arabia; Serology.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures


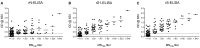

Similar articles
-
Qualitative and Quantitative Determination of MERS-CoV S1-Specific Antibodies Using ELISA.Methods Mol Biol. 2020;2099:127-133. doi: 10.1007/978-1-0716-0211-9_11. Methods Mol Biol. 2020. PMID: 31883093 Free PMC article.
-
Characterization of anti-MERS-CoV antibodies against various recombinant structural antigens of MERS-CoV in an imported case in China.Emerg Microbes Infect. 2016 Nov 9;5(11):e113. doi: 10.1038/emi.2016.114. Emerg Microbes Infect. 2016. PMID: 27826140 Free PMC article.
-
Suggested new breakpoints of anti-MERS-CoV antibody ELISA titers: performance analysis of serologic tests.Eur J Clin Microbiol Infect Dis. 2017 Nov;36(11):2179-2186. doi: 10.1007/s10096-017-3043-3. Epub 2017 Jul 11. Eur J Clin Microbiol Infect Dis. 2017. PMID: 28695355 Free PMC article.
-
Antibodies and vaccines against Middle East respiratory syndrome coronavirus.Emerg Microbes Infect. 2019;8(1):841-856. doi: 10.1080/22221751.2019.1624482. Emerg Microbes Infect. 2019. PMID: 31169078 Free PMC article. Review.
-
MERS-CoV diagnosis: An update.J Infect Public Health. 2016 May-Jun;9(3):216-9. doi: 10.1016/j.jiph.2016.04.005. Epub 2016 Apr 20. J Infect Public Health. 2016. PMID: 27106390 Free PMC article. Review.
Cited by
-
Mucosal SARS-CoV-2 S1 adenovirus-based vaccine elicits robust systemic and mucosal immunity and protects against disease in animals.mBio. 2025 Jan 8;16(1):e0217024. doi: 10.1128/mbio.02170-24. Epub 2024 Dec 4. mBio. 2025. PMID: 39629990 Free PMC article.
-
Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels.Sci Rep. 2019 Nov 8;9(1):16292. doi: 10.1038/s41598-019-52730-4. Sci Rep. 2019. PMID: 31705137 Free PMC article.
-
High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients.J Infect. 2020 Sep;81(3):420-426. doi: 10.1016/j.jinf.2020.05.067. Epub 2020 Jun 4. J Infect. 2020. PMID: 32504745 Free PMC article.
-
Evaluation of MERS-CoV Neutralizing Antibodies in Sera Using Live Virus Microneutralization Assay.Methods Mol Biol. 2020;2099:107-116. doi: 10.1007/978-1-0716-0211-9_9. Methods Mol Biol. 2020. PMID: 31883091 Free PMC article.
-
Qualitative and Quantitative Determination of MERS-CoV S1-Specific Antibodies Using ELISA.Methods Mol Biol. 2020;2099:127-133. doi: 10.1007/978-1-0716-0211-9_11. Methods Mol Biol. 2020. PMID: 31883093 Free PMC article.
References
-
- Agnihothram S., Gopal R., Yount B.L.Jr., Donaldson E.F., Menachery V.D., Graham R.L., Scobey T.D., Gralinski L.E., Denison M.R., Zambon M., Baric R.S. Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J. Infect. Dis. 2014;209:995–1006. - PMC - PubMed
-
- Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R., Al Nsour M., Iblan I., Jarour N., Farag N.H., Haddadin A., Al-Sanouri T., Tamin A., Harcourt J.L., Kuhar D.T., Swerdlow D.L., Erdman D.D., Pallansch M.A., Haynes L.M., Gerber S.I. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin. Infect. Dis. 2014;59:1225–1233. - PMC - PubMed
-
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., Kapoor A., Epstein J.H., Karesh W.B., Daszak P., Mohammed O.B., Lipkin W.I. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5:e00884–e00914. - PMC - PubMed
-
- Alshukairi A.N., Khalid I., Ahmed W.A., Dada A.M., Bayumi D.T., Malic L.S., Althawadi S., Ignacio K., Alsalmi H.S., Al-Abdely H.M., Wali G.Y., Qushmaq I.A., Alraddadi B.M., Perlman S. Antibody response and disease severity in healthcare worker MERS survivors. Emerg. Inf. Dis. 2016;22:1113–1115. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

