Safety of 9-valent human papillomavirus vaccine administration among pregnant women: Adverse event reports in the Vaccine Adverse Event Reporting System (VAERS), 2014-2017
- PMID: 30660400
- PMCID: PMC6505695
- DOI: 10.1016/j.vaccine.2018.11.077
Safety of 9-valent human papillomavirus vaccine administration among pregnant women: Adverse event reports in the Vaccine Adverse Event Reporting System (VAERS), 2014-2017
Abstract
Introduction: 9-valent human papillomavirus vaccine (9vHPV) was approved by the Food and Drug Administration (FDA) in December 2014. 9vHPV is not recommended during pregnancy, but some women of childbearing age may be inadvertently exposed. This study aims to evaluate reports submitted to the Vaccine Adverse Event Reporting System (VAERS) of pregnant women exposed to 9vHPV.
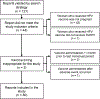
Methods: We searched the VAERS database, a national post-licensure vaccine safety surveillance system, for reports of pregnant women vaccinated with 9vHPV in the United States between December 10, 2014 and December 31, 2017. Disproportionate reporting of adverse events (AEs) was assessed using proportional reporting ratios (PRRs).
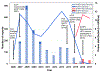
Results: A total of 82 pregnancy reports were identified. Sixty reports (73.2%) did not describe an AE and were submitted only to report the vaccine exposure during pregnancy. The most frequently reported pregnancy-specific AE was spontaneous abortion (n = 3; 3.7%), followed by vaginal bleeding (n = 2; 2.4%). Among non-pregnancy-specific AEs, injection site reaction (n = 3; 3.7%) was most common. No disproportionate reporting of any AE was found.
Discussion: No unexpected AEs were observed among these pregnancy reports.
Keywords: Adverse event; Epidemiology; Human papillomavirus vaccine; Surveillance; Vaccine safety.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
References
-
- Gruber MF. December 10, 2014 Approval letter—GARDASIL 9. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration; 2014.
-
- Centers for Disease Control and Prevention. HPV Vaccine Information for Clinicians. U.S. Department of Health and Human Services; https://www.cdc.gov/hpv/hcp/need-to-know.pdf. Published December 20, 2016. Accessed January 2018.
-
- Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and mortality weekly report. 2016;65(49):1405–8. - PubMed
-
- Merck Sharp & Dohme Corp. Highlights of Prescribing Information Gardasil®9 (Human Papillomavirus 9-valent Vaccine, Recombinant). Merck & Co., Inc; https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedPr.... Published 2014. Updated February 2018. Accessed April 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical